Loss of the vitamin D receptor in human breast and prostate cancers strongly induces cell apoptosis through downregulation of Wnt/β-catenin signaling
- PMID: 28944088
- PMCID: PMC5605769
- DOI: 10.1038/boneres.2017.23
Loss of the vitamin D receptor in human breast and prostate cancers strongly induces cell apoptosis through downregulation of Wnt/β-catenin signaling
Abstract
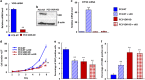
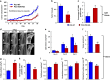
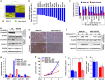
Vitamin D co-regulates cell proliferation, differentiation and apoptosis in numerous tissues, including cancers. The known anti-proliferative and pro-apoptotic actions of the active metabolite of vitamin D, 1,25-dihydroxy-vitamin D [1,25(OH)2D] are mediated through binding to the vitamin D receptor (VDR). Here, we report on the unexpected finding that stable knockdown of VDR expression in the human breast and prostate cancer cell lines, MDA-MB-231 and PC3, strongly induces cell apoptosis and inhibits cell proliferation in vitro. Implantation of these VDR knockdown cells into the mammary fat pad (MDA-MB-231), subcutaneously (PC3) or intra-tibially (both cell lines) in immune-incompetent nude mice resulted in reduced tumor growth associated with increased apoptosis and reduced cell proliferation compared with controls. These growth-retarding effects of VDR knockdown occur in the presence and absence of vitamin D and are independent of whether cells were grown in bone or soft tissues. Transcriptome analysis of VDR knockdown and non-target control cell lines demonstrated that loss of the VDR was associated with significant attenuation in the Wnt/β-catenin signaling pathway. In particular, cytoplasmic and nuclear β-catenin protein levels were reduced with a corresponding downregulation of downstream genes such as Axin2, Cyclin D1, interleukin-6 (IL-6), and IL-8. Stabilization of β-catenin using the GSK-3β inhibitor BIO partly reversed the growth-retarding effects of VDR knockdown. Our results indicate that the unliganded VDR possesses hitherto unknown functions to promote breast and prostate cancer growth, which appear to be operational not only within but also outside the bone environment. These novel functions contrast with the known anti-proliferative nuclear actions of the liganded VDR and may represent targets for new diagnostic and therapeutic approaches in breast and prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The vitamin D receptor is involved in the regulation of human breast cancer cell growth via a ligand-independent function in cytoplasm.Oncotarget. 2017 Apr 18;8(16):26687-26701. doi: 10.18632/oncotarget.15803. Oncotarget. 2017. PMID: 28460457 Free PMC article.
-
Vitamin D-induced vitamin D receptor expression induces tamoxifen sensitivity in MCF-7 stem cells via suppression of Wnt/β-catenin signaling.Biosci Rep. 2018 Dec 7;38(6):BSR20180595. doi: 10.1042/BSR20180595. Print 2018 Dec 21. Biosci Rep. 2018. PMID: 30314996 Free PMC article.
-
Vitamin D3-dependent VDR signaling delays ron-mediated breast tumorigenesis through suppression of β-catenin activity.Oncotarget. 2015 Jun 30;6(18):16304-20. doi: 10.18632/oncotarget.4059. Oncotarget. 2015. PMID: 26008979 Free PMC article.
-
Vitamin D and systemic cancer: is this relevant to malignant melanoma?Br J Dermatol. 2002 Aug;147(2):197-213. doi: 10.1046/j.1365-2133.2002.04960.x. Br J Dermatol. 2002. PMID: 12174089 Review.
-
Targets of vitamin D receptor signaling in the mammary gland.J Bone Miner Res. 2007 Dec;22 Suppl 2:V86-90. doi: 10.1359/jbmr.07s204. J Bone Miner Res. 2007. PMID: 18290729 Review.
Cited by
-
Association of Methylenetetrahydrofolate Reductase, Vitamin D Receptor, and Interleukin-16 Gene Polymorphisms With Renal Cell Carcinoma Risk.Technol Cancer Res Treat. 2019 Jan 1;18:1533033819859413. doi: 10.1177/1533033819859413. Technol Cancer Res Treat. 2019. PMID: 31242814 Free PMC article.
-
Myeloid vitamin D receptor regulates Paneth cells and microbial homeostasis.FASEB J. 2023 Jun;37(6):e22957. doi: 10.1096/fj.202202169RR. FASEB J. 2023. PMID: 37219463 Free PMC article.
-
Skeletal Abnormalities and VDR1 Gene Polymorphisms in Mucopolysaccharidosis Patients.Pharmgenomics Pers Med. 2021 Mar 17;14:349-358. doi: 10.2147/PGPM.S295241. eCollection 2021. Pharmgenomics Pers Med. 2021. PMID: 33889011 Free PMC article.
-
Bone Metastases of Diverse Primary Origin Frequently Express the VDR (Vitamin D Receptor) and CYP24A1.J Clin Med. 2022 Nov 3;11(21):6537. doi: 10.3390/jcm11216537. J Clin Med. 2022. PMID: 36362766 Free PMC article.
-
Vitamin D receptor prevents tumour development by regulating the Wnt/β-catenin signalling pathway in human colorectal cancer.BMC Cancer. 2023 Apr 12;23(1):336. doi: 10.1186/s12885-023-10690-z. BMC Cancer. 2023. PMID: 37046222 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

