Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine
- PMID: 28944281
- PMCID: PMC5609467
- DOI: 10.1016/j.gendis.2017.04.001
Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine
Abstract
With rapid advances in understanding molecular pathogenesis of human diseases in the era of genome sciences and systems biology, it is anticipated that increasing numbers of therapeutic genes or targets will become available for targeted therapies. Despite numerous setbacks, efficacious gene and/or cell-based therapies still hold the great promise to revolutionize the clinical management of human diseases. It is wildly recognized that poor gene delivery is the limiting factor for most in vivo gene therapies. There has been a long-lasting interest in using viral vectors, especially adenoviral vectors, to deliver therapeutic genes for the past two decades. Among all currently available viral vectors, adenovirus is the most efficient gene delivery system in a broad range of cell and tissue types. The applications of adenoviral vectors in gene delivery have greatly increased in number and efficiency since their initial development. In fact, among over 2,000 gene therapy clinical trials approved worldwide since 1989, a significant portion of the trials have utilized adenoviral vectors. This review aims to provide a comprehensive overview on the characteristics of adenoviral vectors, including adenoviral biology, approaches to engineering adenoviral vectors, and their applications in clinical and pre-clinical studies with an emphasis in the areas of cancer treatment, vaccination and regenerative medicine. Current challenges and future directions regarding the use of adenoviral vectors are also discussed. It is expected that the continued improvements in adenoviral vectors should provide great opportunities for cell and gene therapies to live up to its enormous potential in personalized medicine.
Keywords: adenoviral vector; adenovirus; cell therapy; gene delivery; gene therapy; gene transfer; non-viral vectors; oncolytic virus; personalized medicine; regenerative medicine; tissue engineering; vaccine development; viral vectors.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare no conflicts of interest.
Figures
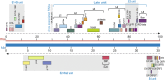
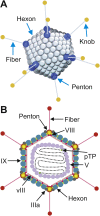




References
-
- Kay M.A., Glorioso J.C., Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7(1):33–40. - PubMed
-
- Breyer B., Jiang W., Cheng H. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1(2):149–162. - PubMed
-
- Seymour L.W., Fisher K.D. Adenovirus: teaching an old dog new tricks. Hum Gene Ther. 2011;22(9):1041–1042. - PubMed
-
- Rauschhuber C., Noske N., Ehrhardt A. New insights into stability of recombinant adenovirus vector genomes in mammalian cells. Eur J Cell Biol. 2012;91(1):2–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
