Metabolic Origins of Heart Failure
- PMID: 28944310
- PMCID: PMC5609457
- DOI: 10.1016/j.jacbts.2016.11.009
Metabolic Origins of Heart Failure
Abstract
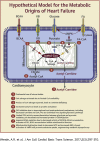
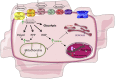
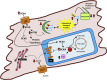
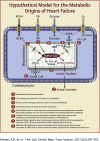
For more than half a century, metabolic perturbations have been explored in the failing myocardium, highlighting a reversion to a more fetal-like metabolic profile (characterized by depressed fatty acid oxidation and concomitant increased reliance on glucose utilization). More recently, alterations in ketone body and amino acid/protein metabolism have been described during heart failure, as well as mitochondrial dysfunction and perturbed metabolic signaling (e.g., acetylation, O-GlcNAcylation). Although numerous mechanisms are likely involved, the current review provides recent advances regarding the metabolic origins of heart failure, and their potential contribution toward contractile dysfunction of the heart.
Keywords: amino acids; fatty acids; glucose; heart failure; ketone bodies.
Figures


Similar articles
-
Loss of Metabolic Flexibility in the Failing Heart.Front Cardiovasc Med. 2018 Jun 6;5:68. doi: 10.3389/fcvm.2018.00068. eCollection 2018. Front Cardiovasc Med. 2018. PMID: 29928647 Free PMC article. Review.
-
Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency.Cardiovasc Res. 2019 Sep 1;115(11):1606-1616. doi: 10.1093/cvr/cvz045. Cardiovasc Res. 2019. PMID: 30778524 Free PMC article.
-
Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart.Biochim Biophys Acta. 2011 Jul;1813(7):1333-50. doi: 10.1016/j.bbamcr.2011.01.015. Epub 2011 Jan 20. Biochim Biophys Acta. 2011. PMID: 21256164 Review.
-
Myocardial Energy Substrate Metabolism in Heart Failure : from Pathways to Therapeutic Targets.Curr Pharm Des. 2015;21(25):3654-64. doi: 10.2174/1381612821666150710150445. Curr Pharm Des. 2015. PMID: 26166604 Review.
-
Acetylation control of cardiac fatty acid β-oxidation and energy metabolism in obesity, diabetes, and heart failure.Biochim Biophys Acta. 2016 Dec;1862(12):2211-2220. doi: 10.1016/j.bbadis.2016.07.020. Epub 2016 Jul 29. Biochim Biophys Acta. 2016. PMID: 27479696 Review.
Cited by
-
Integrated multi-omics analysis of adverse cardiac remodeling and metabolic inflexibility upon ErbB2 and ERRα deficiency.Commun Biol. 2022 Sep 12;5(1):955. doi: 10.1038/s42003-022-03942-4. Commun Biol. 2022. PMID: 36097051 Free PMC article.
-
Semantic Multi-Classifier Systems Identify Predictive Processes in Heart Failure Models across Species.Biomolecules. 2018 Nov 26;8(4):158. doi: 10.3390/biom8040158. Biomolecules. 2018. PMID: 30486323 Free PMC article.
-
Left ventricular remodeling response to SGLT2 inhibitors in heart failure: an updated meta-analysis of randomized controlled studies.Cardiovasc Diabetol. 2023 Sep 2;22(1):235. doi: 10.1186/s12933-023-01970-w. Cardiovasc Diabetol. 2023. PMID: 37660005 Free PMC article.
-
Metabolic remodeling of substrate utilization during heart failure progression.Heart Fail Rev. 2019 Jan;24(1):143-154. doi: 10.1007/s10741-018-9713-0. Heart Fail Rev. 2019. PMID: 29789980
-
Time of eating and mortality in U.S. adults with heart failure: Analyses of the National Health and Nutrition Examination Survey 2003-2018.Nutr Metab Cardiovasc Dis. 2024 Feb;34(2):445-454. doi: 10.1016/j.numecd.2023.10.013. Epub 2023 Oct 14. Nutr Metab Cardiovasc Dis. 2024. PMID: 38155047 Free PMC article.
References
-
- Bers D.M. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol. 2014;76:107–127. - PubMed
-
- Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. - PubMed
-
- Allard M., Schonekess B., Henning S., English D., Lopaschuk G. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. - PubMed
-
- Goodwin G., Taylor C., Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

