Microsporidia: Obligate Intracellular Pathogens Within the Fungal Kingdom
- PMID: 28944750
- PMCID: PMC5613672
- DOI: 10.1128/microbiolspec.FUNK-0018-2016
Microsporidia: Obligate Intracellular Pathogens Within the Fungal Kingdom
Abstract
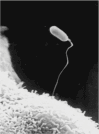
Microsporidia are obligate intracellular pathogens related to Fungi. These organisms have a unique invasion organelle, the polar tube, which upon appropriate environmental stimulation rapidly discharges out of the spore, pierces a host cell's membrane, and serves as a conduit for sporoplasm passage into the host cell. Phylogenetic analysis suggests that microsporidia are related to the Fungi, being either a basal branch or sister group. Despite the description of microsporidia over 150 years ago, we still lack an understanding of the mechanism of invasion, including the role of various polar tube proteins, spore wall proteins, and host cell proteins in the formation and function of the invasion synapse. Recent advances in ultrastructural techniques are helping to better define the formation and functioning of the invasion synapse. Over the past 2 decades, proteomic approaches have helped define polar tube proteins and spore wall proteins as well as the importance of posttranslational modifications such as glycosylation in the functioning of these proteins, but the absence of genetic techniques for the manipulation of microsporidia has hampered research on the function of these various proteins. The study of the mechanism of invasion should provide fundamental insights into the biology of these ubiquitous intracellular pathogens that can be integrated into studies aimed at treating or controlling microsporidiosis.
Figures


Similar articles
-
The microsporidian polar tube: a highly specialised invasion organelle.Int J Parasitol. 2005 Aug;35(9):941-53. doi: 10.1016/j.ijpara.2005.04.003. Int J Parasitol. 2005. PMID: 16005007 Free PMC article. Review.
-
The Function and Structure of the Microsporidia Polar Tube.Exp Suppl. 2022;114:179-213. doi: 10.1007/978-3-030-93306-7_8. Exp Suppl. 2022. PMID: 35544004 Free PMC article.
-
Microsporidia: emerging pathogenic protists.Acta Trop. 2001 Feb 23;78(2):89-102. doi: 10.1016/s0001-706x(00)00178-9. Acta Trop. 2001. PMID: 11230819 Review.
-
The roles of microsporidia spore wall proteins in the spore wall formation and polar tube anchorage to spore wall during development and infection processes.Exp Parasitol. 2018 Apr;187:93-100. doi: 10.1016/j.exppara.2018.03.007. Epub 2018 Mar 6. Exp Parasitol. 2018. PMID: 29522765 Review.
-
New insights into Microsporidia polar tube function and invasion mechanism.J Eukaryot Microbiol. 2024 Sep-Oct;71(5):e13043. doi: 10.1111/jeu.13043. Epub 2024 Jul 7. J Eukaryot Microbiol. 2024. PMID: 38973152 Review.
Cited by
-
MicroAnnot: A Dedicated Workflow for Accurate Microsporidian Genome Annotation.Int J Mol Sci. 2024 Jan 10;25(2):880. doi: 10.3390/ijms25020880. Int J Mol Sci. 2024. PMID: 38255958 Free PMC article.
-
North American Propolis Extracts From Upstate New York Decrease Nosema ceranae (Microsporidia) Spore Levels in Honey Bees (Apis mellifera).Front Microbiol. 2020 Jul 22;11:1719. doi: 10.3389/fmicb.2020.01719. eCollection 2020. Front Microbiol. 2020. PMID: 32793168 Free PMC article.
-
Current Therapy and Therapeutic Targets for Microsporidiosis.Front Microbiol. 2022 Mar 9;13:835390. doi: 10.3389/fmicb.2022.835390. eCollection 2022. Front Microbiol. 2022. PMID: 35356517 Free PMC article. Review.
-
Generation of a Microsporidia Species Attribute Database and Analysis of the Extensive Ecological and Phenotypic Diversity of Microsporidia.mBio. 2021 Jun 29;12(3):e0149021. doi: 10.1128/mBio.01490-21. Epub 2021 Jun 29. mBio. 2021. PMID: 34182782 Free PMC article.
-
The genome of an intranuclear parasite, Paramicrosporidium saccamoebae, reveals alternative adaptations to obligate intracellular parasitism.Elife. 2017 Nov 24;6:e29594. doi: 10.7554/eLife.29594. Elife. 2017. PMID: 29171834 Free PMC article.
References
-
- Pasteur L. 1870. Études sur la maladie des vers à soie [M]. Paris, Gauthier-Villars, successeur de Mallet-Bachelier 1870:148–168.
-
- Weiss LM, Becnel JJ (ed). 2014. Microsporidia: Pathogens of Opportunity. Wiley-Blackwell, Oxford, United Kingdom. 10.1002/9781118395264 - DOI
-
- Fries I. 1993. Nosema apis: a parasite in the honey bee colony. Bee World 74:5–19. 10.1080/0005772X.1993.11099149 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

