Asymmetry of movements in CFTR's two ATP sites during pore opening serves their distinct functions
- PMID: 28944753
- PMCID: PMC5626490
- DOI: 10.7554/eLife.29013
Asymmetry of movements in CFTR's two ATP sites during pore opening serves their distinct functions
Abstract
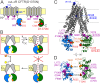
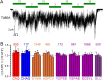
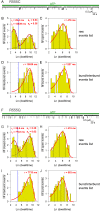
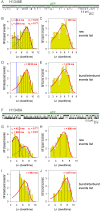
CFTR, the chloride channel mutated in cystic fibrosis (CF) patients, is opened by ATP binding to two cytosolic nucleotide binding domains (NBDs), but pore-domain mutations may also impair gating. ATP-bound NBDs dimerize occluding two nucleotides at interfacial binding sites; one site hydrolyzes ATP, the other is inactive. The pore opens upon tightening, and closes upon disengagement, of the catalytic site following ATP hydrolysis. Extent, timing, and role of non-catalytic-site movements are unknown. Here we exploit equilibrium gating of a hydrolysis-deficient mutant and apply Φ value analysis to compare timing of opening-associated movements at multiple locations, from the cytoplasmic ATP sites to the extracellular surface. Marked asynchrony of motion in the two ATP sites reveals their distinct roles in channel gating. The results clarify the molecular mechanisms of functional cross-talk between canonical and degenerate ATP sites in asymmetric ABC proteins, and of the gating defects caused by two common CF mutations.
Keywords: E. coli; R117H; REFER; asymmetric ABC protein; biophysics; cystic fibrosis; deltaF508; structural biology; xenopus.
Conflict of interest statement
Reviewing Editor, eLife.
No competing interests declared.
Figures




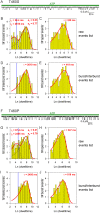

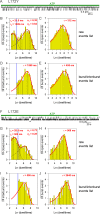
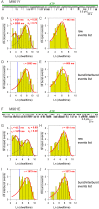
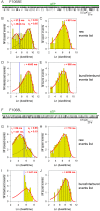



Similar articles
-
The two ATP binding sites of cystic fibrosis transmembrane conductance regulator (CFTR) play distinct roles in gating kinetics and energetics.J Gen Physiol. 2006 Oct;128(4):413-22. doi: 10.1085/jgp.200609622. Epub 2006 Sep 11. J Gen Physiol. 2006. PMID: 16966475 Free PMC article.
-
Mutant cycles at CFTR's non-canonical ATP-binding site support little interface separation during gating.J Gen Physiol. 2011 Jun;137(6):549-62. doi: 10.1085/jgp.201110608. Epub 2011 May 16. J Gen Physiol. 2011. PMID: 21576373 Free PMC article.
-
Structure-activity analysis of a CFTR channel potentiator: Distinct molecular parts underlie dual gating effects.J Gen Physiol. 2014 Oct;144(4):321-36. doi: 10.1085/jgp.201411246. J Gen Physiol. 2014. PMID: 25267914 Free PMC article.
-
Gating of the CFTR Cl- channel by ATP-driven nucleotide-binding domain dimerisation.J Physiol. 2009 May 15;587(Pt 10):2151-61. doi: 10.1113/jphysiol.2009.171595. Epub 2009 Mar 30. J Physiol. 2009. PMID: 19332488 Free PMC article. Review.
-
STRUCTURE, GATING, AND REGULATION OF THE CFTR ANION CHANNEL.Physiol Rev. 2019 Jan 1;99(1):707-738. doi: 10.1152/physrev.00007.2018. Physiol Rev. 2019. PMID: 30516439 Review.
Cited by
-
Directed Mutational Strategies Reveal Drug Binding and Transport by the MDR Transporters of Candida albicans.J Fungi (Basel). 2021 Jan 20;7(2):68. doi: 10.3390/jof7020068. J Fungi (Basel). 2021. PMID: 33498218 Free PMC article. Review.
-
CRISPR/Cas9 bioluminescence-based assay for monitoring CFTR trafficking to the plasma membrane.Life Sci Alliance. 2023 Nov 2;7(1):e202302045. doi: 10.26508/lsa.202302045. Print 2024 Jan. Life Sci Alliance. 2023. PMID: 37918963 Free PMC article.
-
Computational analysis of long-range allosteric communications in CFTR.Elife. 2023 Dec 18;12:RP88659. doi: 10.7554/eLife.88659. Elife. 2023. PMID: 38109179 Free PMC article.
-
Identifying the molecular target sites for CFTR potentiators GLPG1837 and VX-770.J Gen Physiol. 2019 Jul 1;151(7):912-928. doi: 10.1085/jgp.201912360. Epub 2019 Jun 4. J Gen Physiol. 2019. PMID: 31164398 Free PMC article.
-
Physiological and pharmacological characterization of the N1303K mutant CFTR.J Cyst Fibros. 2018 Sep;17(5):573-581. doi: 10.1016/j.jcf.2018.05.011. Epub 2018 Jun 7. J Cyst Fibros. 2018. PMID: 29887518 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

