Targeting microbial biofilms: current and prospective therapeutic strategies
- PMID: 28944770
- PMCID: PMC5685531
- DOI: 10.1038/nrmicro.2017.99
Targeting microbial biofilms: current and prospective therapeutic strategies
Abstract
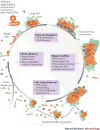
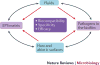
Biofilm formation is a key virulence factor for a wide range of microorganisms that cause chronic infections. The multifactorial nature of biofilm development and drug tolerance imposes great challenges for the use of conventional antimicrobials and indicates the need for multi-targeted or combinatorial therapies. In this Review, we focus on current therapeutic strategies and those under development that target vital structural and functional traits of microbial biofilms and drug tolerance mechanisms, including the extracellular matrix and dormant cells. We emphasize strategies that are supported by in vivo or ex vivo studies, highlight emerging biofilm-targeting technologies and provide a rationale for multi-targeted therapies aimed at disrupting the complex biofilm microenvironment.
Conflict of interest statement
The authors declare competing interests.
Figures


References
-
- Magin CM, Cooper SP, Brennan AB. Non-toxic antifouling strategies. Materials Today. 2010;13:36–44.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

