Enhanced immune response induced by P5 HER2/neu-derived peptide-pulsed dendritic cells as a preventive cancer vaccine
- PMID: 28944998
- PMCID: PMC5742681
- DOI: 10.1111/jcmm.13343
Enhanced immune response induced by P5 HER2/neu-derived peptide-pulsed dendritic cells as a preventive cancer vaccine
Abstract
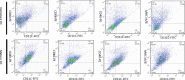
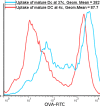
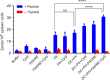
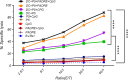
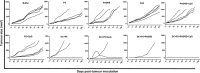
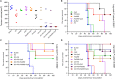
Dendritic cells are special and powerful antigen-presenting cells that can induce primary immune responses against tumour-associated antigens. They can present antigens via both MHC-I and MHC-II, so they have the ability to stimulate both cytotoxic T lymphocytes and T helper cells. Furthermore, CD8+ cytotoxic T lymphocytes require activation by CD4+ T cells. This requires a CD4+ T cell activator molecule, of which PADRE is one of the best. We chose an approach to use both of these important arms of the immune system. We prepared dendritic cells from mouse bone marrow, loaded them with our target peptides (P5 peptide alone or P5 + PADRE), and then injected these pulsed dendritic cells alone or in combination with CpG-ODN (as adjuvant) into BALB/C mice. After the last boosting dose, mice were inoculated with TUBO cells, which overexpress HER2/neu. Two weeks after the tumour cell injection, immunological tests were performed on splenocyte suspensions, and the remaining mice were evaluated for tumour growth and survival. Our data indicate the formulation that contains PADRE plus P5 loaded onto DC in combination with CpG-ODN was the most effective formulation at inducing immune responses. Interferon production in CD4+ and CD8+ gated cells, cytotoxicity rates of target cells and mice survival were all significantly greater in this group than in controls, and all the mice in this group were tumour-free throughout the experiment. Based on our results and the role of HER2/neu as a candidate in human immunotherapy, this approach may be an effective cancer treatment.
Keywords: cancer; dendritic Cells; pan HLA-DR epitope peptide; peptide vaccine.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
MPL nano-liposomal vaccine containing P5 HER2/neu-derived peptide pulsed PADRE as an effective vaccine in a mice TUBO model of breast cancer.J Control Release. 2019 Jun 10;303:223-236. doi: 10.1016/j.jconrel.2019.04.019. Epub 2019 Apr 15. J Control Release. 2019. PMID: 30999007
-
Nanoliposomal vaccine containing long multi-epitope peptide E75-AE36 pulsed PADRE-induced effective immune response in mice TUBO model of breast cancer.Eur J Cancer. 2020 Apr;129:80-96. doi: 10.1016/j.ejca.2020.01.010. Epub 2020 Mar 4. Eur J Cancer. 2020. PMID: 32145473
-
Effective induction of anti-tumor immunity using p5 HER-2/neu derived peptide encapsulated in fusogenic DOTAP cationic liposomes co-administrated with CpG-ODN.Immunol Lett. 2014 Nov;162(1 Pt A):87-93. doi: 10.1016/j.imlet.2014.07.008. Epub 2014 Jul 30. Immunol Lett. 2014. PMID: 25086399
-
Dendritic cell gene therapy.Surg Oncol Clin N Am. 2002 Jul;11(3):645-60. doi: 10.1016/s1055-3207(02)00027-3. Surg Oncol Clin N Am. 2002. PMID: 12487060 Review.
-
Participation of T cells in generating immune protection against cancers.Pathol Res Pract. 2024 Oct;262:155534. doi: 10.1016/j.prp.2024.155534. Epub 2024 Aug 12. Pathol Res Pract. 2024. PMID: 39180801 Review.
Cited by
-
Cancer immunotherapy: a promising dawn in cancer research.Am J Blood Res. 2020 Dec 15;10(6):375-385. eCollection 2020. Am J Blood Res. 2020. PMID: 33489447 Free PMC article. Review.
-
Dendritic Cell Tumor Vaccination via Fc Gamma Receptor Targeting: Lessons Learned from Pre-Clinical and Translational Studies.Vaccines (Basel). 2021 Apr 20;9(4):409. doi: 10.3390/vaccines9040409. Vaccines (Basel). 2021. PMID: 33924183 Free PMC article. Review.
-
Recent Advances in Therapeutic Peptides: Innovations and Applications in Treating Infections and Diseases.ACS Omega. 2025 Apr 23;10(17):17087-17107. doi: 10.1021/acsomega.5c02077. eCollection 2025 May 6. ACS Omega. 2025. PMID: 40352490 Free PMC article. Review.
-
PD-1 blockade enhances radio-immunotherapy efficacy in murine tumor models.J Cancer Res Clin Oncol. 2018 Oct;144(10):1909-1920. doi: 10.1007/s00432-018-2723-4. Epub 2018 Aug 3. J Cancer Res Clin Oncol. 2018. PMID: 30074066 Free PMC article.
-
Overexpression of Apolipoprotein C1 (APOC1) in Clear Cell Renal Cell Carcinoma and Its Prognostic Significance.Med Sci Monit. 2021 Feb 16;27:e929347. doi: 10.12659/MSM.929347. Med Sci Monit. 2021. PMID: 33591959 Free PMC article.
References
-
- Wei H, Wang S, Zhang D, et al Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin Cancer Res. 2009; 15: 4612–21. - PubMed
-
- Koido S, Hara E, Homma S, et al Dendritic cells fused with allogeneic colorectal cancer cell line present multiple colorectal cancer‐specific antigens and induce antitumor immunity against autologous tumor cells. Clin Cancer Res. 2005; 11: 7891–900. - PubMed
-
- Tokunaga N, Murakami T, Endo Y, et al Human monocyte‐derived dendritic cells pulsed with wild‐type p53 protein efficiently induce CTLs against p53 overexpressing human cancer cells. Clin Cancer Res. 2005; 11: 1312–8. - PubMed
-
- Shariat S, Badiee A, Jalali SA, et al P5 HER2/neu‐derived peptide conjugated to liposomes containing MPL adjuvant as an effective prophylactic vaccine formulation for breast cancer. Cancer Lett. 2014; 355: 54–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

