Two-photon calcium imaging of the medial prefrontal cortex and hippocampus without cortical invasion
- PMID: 28945191
- PMCID: PMC5643091
- DOI: 10.7554/eLife.26839
Two-photon calcium imaging of the medial prefrontal cortex and hippocampus without cortical invasion
Abstract
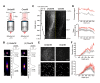
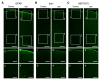
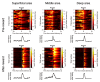
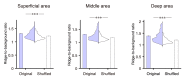
In vivo two-photon calcium imaging currently allows us to observe the activity of multiple neurons up to ~900 µm below the cortical surface without cortical invasion. However, many important brain areas are located deeper than this. Here, we used an 1100 nm laser that underfilled the back aperture of the objective together with red genetically encoded calcium indicators to establish two-photon calcium imaging of the intact mouse brain and detect neural activity up to 1200 μm from the cortical surface. This imaging was obtained from the medial prefrontal cortex (the prelimbic area) and the hippocampal CA1 region. We found that neural activity before water delivery repeated at a constant interval was higher in the prelimbic area than in layer 2/3 of the secondary motor area. Reducing the invasiveness of imaging is an important strategy to reveal the intact brain processes active in cognition and memory.
Keywords: Hippocampus; Prefrontal cortex; Two-photon imaging; mouse; neuroscience.
Conflict of interest statement
No competing interests declared.
Figures









Similar articles
-
A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging.Elife. 2016 Jun 14;5:e14472. doi: 10.7554/eLife.14472. Elife. 2016. PMID: 27300105 Free PMC article.
-
Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey.Hippocampus. 1995;5(6):511-33. doi: 10.1002/hipo.450050604. Hippocampus. 1995. PMID: 8646279
-
Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics.Hippocampus. 2000;10(4):411-9. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. Hippocampus. 2000. PMID: 10985280 Review.
-
In Vivo Two-Photon Calcium Imaging of Hippocampal Neurons in Alzheimer Mouse Models.Methods Mol Biol. 2018;1750:341-351. doi: 10.1007/978-1-4939-7704-8_23. Methods Mol Biol. 2018. PMID: 29512084
-
The need for calcium imaging in nonhuman primates: New motor neuroscience and brain-machine interfaces.Exp Neurol. 2017 Jan;287(Pt 4):437-451. doi: 10.1016/j.expneurol.2016.08.003. Epub 2016 Aug 7. Exp Neurol. 2017. PMID: 27511294 Free PMC article. Review.
Cited by
-
All-fiber dissipative soliton Raman laser based on phosphosilicate fiber.IEEE Photonics Technol Lett. 2018 Nov;30(21):1846-1849. doi: 10.1109/LPT.2018.2868070. Epub 2018 Oct 24. IEEE Photonics Technol Lett. 2018. PMID: 30602920 Free PMC article.
-
Three-photon in vivo imaging of neurons and glia in the medial prefrontal cortex with sub-cellular resolution.Commun Biol. 2025 May 23;8(1):795. doi: 10.1038/s42003-025-08079-8. Commun Biol. 2025. PMID: 40410447 Free PMC article.
-
PEO-CYTOP Fluoropolymer Nanosheets as a Novel Open-Skull Window for Imaging of the Living Mouse Brain.iScience. 2020 Sep 21;23(10):101579. doi: 10.1016/j.isci.2020.101579. eCollection 2020 Oct 23. iScience. 2020. PMID: 33083745 Free PMC article.
-
Direct wavefront sensing enables functional imaging of infragranular axons and spines.Nat Methods. 2019 Jul;16(7):615-618. doi: 10.1038/s41592-019-0434-7. Epub 2019 Jun 17. Nat Methods. 2019. PMID: 31209383 Free PMC article.
-
Two-photon calcium imaging of neuronal activity.Nat Rev Methods Primers. 2022;2(1):67. doi: 10.1038/s43586-022-00147-1. Epub 2022 Sep 1. Nat Rev Methods Primers. 2022. PMID: 38124998 Free PMC article.
References
-
- Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, Patel R, Macklin JJ, Bargmann CI, Ahrens MB, Schreiter ER, Jayaraman V, Looger LL, Svoboda K, Kim DS. Sensitive red protein calcium indicators for imaging neural activity. eLife. 2016;5:413. doi: 10.7554/eLife.12727. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

