Mutations in the netrin-1 gene cause congenital mirror movements
- PMID: 28945198
- PMCID: PMC5663368
- DOI: 10.1172/JCI95442
Mutations in the netrin-1 gene cause congenital mirror movements
Abstract
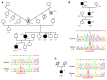
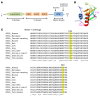
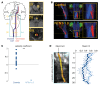
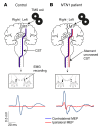
Netrin-1 is a secreted protein that was first identified 20 years ago as an axon guidance molecule that regulates midline crossing in the CNS. It plays critical roles in various tissues throughout development and is implicated in tumorigenesis and inflammation in adulthood. Despite extensive studies, no inherited human disease has been directly associated with mutations in NTN1, the gene coding for netrin-1. Here, we have identified 3 mutations in exon 7 of NTN1 in 2 unrelated families and 1 sporadic case with isolated congenital mirror movements (CMM), a disorder characterized by involuntary movements of one hand that mirror intentional movements of the opposite hand. Given the diverse roles of netrin-1, the absence of manifestations other than CMM in NTN1 mutation carriers was unexpected. Using multimodal approaches, we discovered that the anatomy of the corticospinal tract (CST) is abnormal in patients with NTN1-mutant CMM. When expressed in HEK293 or stable HeLa cells, the 3 mutated netrin-1 proteins were almost exclusively detected in the intracellular compartment, contrary to WT netrin-1, which is detected in both intracellular and extracellular compartments. Since netrin-1 is a diffusible extracellular cue, the pathophysiology likely involves its loss of function and subsequent disruption of axon guidance, resulting in abnormal decussation of the CST.
Conflict of interest statement
Figures


Similar articles
-
Ventricular Netrin-1 deficiency leads to defective pyramidal decussation and mirror movement in mice.Cell Death Dis. 2024 May 17;15(5):343. doi: 10.1038/s41419-024-06719-1. Cell Death Dis. 2024. PMID: 38760361 Free PMC article.
-
Defining the Genetic Landscape of Congenital Mirror Movements in 80 Affected Individuals.Mov Disord. 2024 Feb;39(2):400-410. doi: 10.1002/mds.29669. Epub 2024 Feb 5. Mov Disord. 2024. PMID: 38314870
-
RAD51 haploinsufficiency causes congenital mirror movements in humans.Am J Hum Genet. 2012 Feb 10;90(2):301-7. doi: 10.1016/j.ajhg.2011.12.002. Epub 2012 Feb 2. Am J Hum Genet. 2012. PMID: 22305526 Free PMC article.
-
DCC mutation update: Congenital mirror movements, isolated agenesis of the corpus callosum, and developmental split brain syndrome.Hum Mutat. 2018 Jan;39(1):23-39. doi: 10.1002/humu.23361. Epub 2017 Nov 11. Hum Mutat. 2018. PMID: 29068161 Free PMC article. Review.
-
Congenital mirror movements: a clue to understanding bimanual motor control.J Neurol. 2011 Nov;258(11):1911-9. doi: 10.1007/s00415-011-6107-9. Epub 2011 Jun 3. J Neurol. 2011. PMID: 21633904 Review.
Cited by
-
Locomotor recovery after spinal cord injury: intimate dependence between axonal regeneration and re-connection.Neural Regen Res. 2022 Mar;17(3):553-554. doi: 10.4103/1673-5374.320977. Neural Regen Res. 2022. PMID: 34380886 Free PMC article. No abstract available.
-
Smoothened overexpression causes trochlear motoneurons to reroute and innervate ipsilateral eyes.Cell Tissue Res. 2021 Apr;384(1):59-72. doi: 10.1007/s00441-020-03352-0. Epub 2021 Jan 6. Cell Tissue Res. 2021. PMID: 33409653 Free PMC article.
-
De Novo Pathogenic Variants in N-cadherin Cause a Syndromic Neurodevelopmental Disorder with Corpus Collosum, Axon, Cardiac, Ocular, and Genital Defects.Am J Hum Genet. 2019 Oct 3;105(4):854-868. doi: 10.1016/j.ajhg.2019.09.005. Am J Hum Genet. 2019. PMID: 31585109 Free PMC article.
-
Corticospinal Tract Development, Evolution, and Skilled Movements.Mov Disord. 2025 Jul;40(7):1221-1232. doi: 10.1002/mds.30199. Epub 2025 Apr 25. Mov Disord. 2025. PMID: 40277091 Free PMC article. Review.
-
Identification, molecular characterization, and in silico structural analysis of larval salivary glands Netrin-A as a potent biomarker from Lucilia sericata (Diptera: Calliphoridae).Genetica. 2022 Dec;150(6):379-394. doi: 10.1007/s10709-022-00164-8. Epub 2022 Sep 22. Genetica. 2022. PMID: 36136258
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

