Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones
- PMID: 28945199
- PMCID: PMC5663366
- DOI: 10.1172/JCI93396
Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones
Abstract
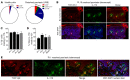
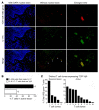
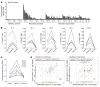
In psoriasis, an IL-17-mediated inflammatory skin disease, skin lesions resolve with therapy, but often recur in the same locations when therapy is discontinued. We propose that residual T cell populations in resolved psoriatic lesions represent the pathogenic T cells of origin in this disease. Utilizing high-throughput screening (HTS) of the T cell receptor (TCR) and immunostaining, we found that clinically resolved psoriatic lesions contained oligoclonal populations of T cells that produced IL-17A in both resolved and active psoriatic lesions. Putative pathogenic clones preferentially utilized particular Vβ and Vα subfamilies. We identified 15 TCRβ and 4 TCRα antigen receptor sequences shared between psoriasis patients and not observed in healthy controls or other inflammatory skin conditions. To address the relative roles of αβ versus γδ T cells in psoriasis, we carried out TCR/δ HTS. These studies demonstrated that the majority of T cells in psoriasis and healthy skin are αβ T cells. γδ T cells made up 1% of T cells in active psoriasis, less than 1% in resolved psoriatic lesions, and less than 2% in healthy skin. All of the 70 most frequent putative pathogenic T cell clones were αβ T cells. In summary, IL-17-producing αβ T cell clones with psoriasis-specific antigen receptors exist in clinically resolved psoriatic skin lesions. These cells likely represent the disease-initiating pathogenic T cells in psoriasis, suggesting that lasting control of this disease will require suppression of these resident T cell populations.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

