TNFα-YAP/p65-HK2 axis mediates breast cancer cell migration
- PMID: 28945218
- PMCID: PMC5623908
- DOI: 10.1038/oncsis.2017.83
TNFα-YAP/p65-HK2 axis mediates breast cancer cell migration
Abstract
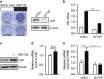
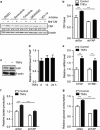
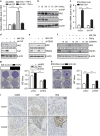
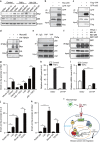
Clinical and experimental evidence indicates that macrophages could promote solid-tumor progression and metastasis. However, the mechanisms underlying this process remain unclear. Here we show that yes-associated protein 1 (YAP1), a transcriptional regulator that controls tissue growth and regeneration, has an important role in tumor necrosis factor α (TNF α)-induced breast cancer migration. Mechanistically, macrophage conditioned medium (CM) or TNFα triggers IκB kinases (IKKs)-mediated YAP phosphorylation and activation in breast cancer cells. We further found that TNFα or macrophage CM treatment increases the interaction between p65 and YAP. Chromatin immunoprecipitation (ChIP) assay shows that YAP/TEAD (TEA domain family member) and p65 proteins synergistically regulate the transcription of hexokinase 2 (HK2), a speed-limiting enzyme in glycolysis, and promotes TNFα-induced or macrophage CM-induced cell migration. Together, our findings indicate an important role of TNFα-IKK-YAP/p65-HK2 signaling axis in the process of inflammation-driven migration in breast cancer cells, which reveals a new molecular link between inflammation and breast cancer metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
AKT2 phosphorylation of hexokinase 2 at T473 promotes tumorigenesis and metastasis in colon cancer cells via NF-κB, HIF1α, MMP2, and MMP9 upregulation.Cell Signal. 2019 Jun;58:99-110. doi: 10.1016/j.cellsig.2019.03.011. Epub 2019 Mar 12. Cell Signal. 2019. PMID: 30877036
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Up-regulation of OLR1 expression by TBC1D3 through activation of TNFα/NF-κB pathway promotes the migration of human breast cancer cells.Cancer Lett. 2017 Nov 1;408:60-70. doi: 10.1016/j.canlet.2017.08.021. Epub 2017 Aug 24. Cancer Lett. 2017. PMID: 28844714
-
Autophagy promotes triple negative breast cancer metastasis via YAP nuclear localization.Biochem Biophys Res Commun. 2019 Dec 3;520(2):263-268. doi: 10.1016/j.bbrc.2019.09.133. Epub 2019 Oct 5. Biochem Biophys Res Commun. 2019. PMID: 31590917
-
Up-regulated ANP32E promotes the thyroid carcinoma cell proliferation and migration via activating AKT/mTOR/HK2-mediated glycolysis.Gene. 2020 Aug 5;750:144681. doi: 10.1016/j.gene.2020.144681. Epub 2020 Apr 15. Gene. 2020. PMID: 32304784
Cited by
-
HTLV-1 activates YAP via NF-κB/p65 to promote oncogenesis.Proc Natl Acad Sci U S A. 2022 Mar 1;119(9):e2115316119. doi: 10.1073/pnas.2115316119. Proc Natl Acad Sci U S A. 2022. PMID: 35210364 Free PMC article.
-
Silencing GMPPB Inhibits the Proliferation and Invasion of GBM via Hippo/MMP3 Pathways.Int J Mol Sci. 2023 Sep 28;24(19):14707. doi: 10.3390/ijms241914707. Int J Mol Sci. 2023. PMID: 37834154 Free PMC article.
-
MicroRNA-216b targets HK2 to potentiate autophagy and apoptosis of breast cancer cells via the mTOR signaling pathway.Int J Biol Sci. 2021 Jul 13;17(11):2970-2983. doi: 10.7150/ijbs.48933. eCollection 2021. Int J Biol Sci. 2021. PMID: 34345220 Free PMC article.
-
WBP2 promotes BTRC mRNA stability to drive migration and invasion in triple-negative breast cancer via NF-κB activation.Mol Oncol. 2022 Jan;16(2):422-446. doi: 10.1002/1878-0261.13048. Epub 2021 Aug 12. Mol Oncol. 2022. PMID: 34197030 Free PMC article.
-
Hsa_circRNA_0040462: a sensor of cells' response to CAP treatment with double-edged roles on breast cancer malignancy.Int J Med Sci. 2022 Mar 21;19(4):640-650. doi: 10.7150/ijms.66940. eCollection 2022. Int J Med Sci. 2022. PMID: 35582416 Free PMC article.
References
-
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012; 125: 5591–5596. - PubMed
-
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4: 71–78. - PubMed
-
- Miles DW, Happerfield LC, Naylor MS, Bobrow LG, Rubens RD, Balkwill FR. Expression of tumour necrosis factor (TNF alpha) and its receptors in benign and malignant breast tissue. Int J Cancer 1994; 56: 777–782. - PubMed
-
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9: 361–371. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

