PTEN deficiency sensitizes endometrioid endometrial cancer to compound PARP-PI3K inhibition but not PARP inhibition as monotherapy
- PMID: 28945226
- PMCID: PMC5799770
- DOI: 10.1038/onc.2017.326
PTEN deficiency sensitizes endometrioid endometrial cancer to compound PARP-PI3K inhibition but not PARP inhibition as monotherapy
Abstract
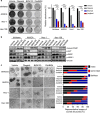
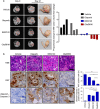
Poly (ADP-ribose) polymerase (PARP) inhibitors have emerged as promising cancer therapeutics especially for tumors with deficient homologous recombination (HR) repair. However, as HR-deficient tumors represent only a small fraction of endometrial cancers, the therapeutic utility of PARP inhibitors is limited in this disease. Somatic loss of phosphatase and tensin homolog (PTEN), a tumor suppressor that counteracts phosphoinositide 3-kinase (PI3K) activity, is one of the most common genetic aberrations in endometrioid endometrial cancer. While previous works have identified the role of PTEN in DNA double-strand break repair, vulnerabilities of PTEN-deficient endometrioid endometrial cancers to PARP inhibition remain controversial. Here we find that PTEN-deficient endometrioid endometrial cancer cells are not responsive to PARP inhibitor Olaparib alone, but instead show superior sensitivity to compound inhibition with PI3K inhibitor BKM120, as evidenced by reduced clonogenic cell growth and three-dimensional (3D) spheroid disintegration. Mechanistically, PI3K blockade by BKM120 attenuated HR competency with γH2AX accumulation and reduced RAD51 and BRCA1 expression in Ishikawa, AN3CA and Nou-1 cells, but the same combination treatment led to enhanced phosphorylation of DNA-PK, a non-homologous end joining repair protein, in Hec-108 cells. Furthermore, we show that CRISPR/Cas9-mediated PTEN depletion rendered PTEN wild-type Hec-1A endometrioid endometrial cancer cells responsive to combined inhibition of PARP/PI3K, with concomitantly induced DNA damage accumulation and repair defects. The combination of BKM120 and Olaparib cooperated to inhibit tumor growth in a genetic mouse model of Pten-deficient endometrioid endometrial cancer. Together, these results suggest PI3K inhibition may be a plausible approach to expand the utility of PARP inhibitors to endometrioid endometrial cancers in a PTEN-deficient setting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet 2005; 366: 491–505. - PubMed
-
- Cancer Genome Atlas Research NCancer Genome Atlas Research NKandoth C Cancer Genome Atlas Research NSchultz N Cancer Genome Atlas Research NCherniack AD Cancer Genome Atlas Research NAkbani R Cancer Genome Atlas Research NLiu Y et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013; 497: 67–73. - PMC - PubMed
-
- Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol 2011; 8: 261–271. - PubMed
-
- Kim G, Ison G, McKee AE, Zhang H, Tang S, Gwise T et al. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res 2015; 21: 4257–4261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

