VPAC1 and VPAC2 receptor activation on GABA release from hippocampal nerve terminals involve several different signalling pathways
- PMID: 28945273
- PMCID: PMC5727335
- DOI: 10.1111/bph.14051
VPAC1 and VPAC2 receptor activation on GABA release from hippocampal nerve terminals involve several different signalling pathways
Abstract
Background and purpose: Vasoactive intestinal peptide (VIP) is an important modulator of hippocampal synaptic transmission that influences both GABAergic synaptic transmission and glutamatergic cell excitability through activation of VPAC1 and VPAC2 receptors. Presynaptic enhancement of GABA release contributes to VIP modulation of hippocampal synaptic transmission.
Experimental approach: We investigated which VIP receptors and coupled transduction pathways were involved in VIP enhancement of K+ -evoked [3 H]-GABA release from isolated nerve terminals of rat hippocampus.
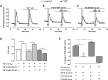
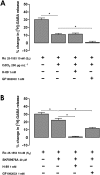
Key results: VIP enhancement of [3 H]-GABA release was potentiated in the presence of the VPAC1 receptor antagonist PG 97-269 but converted into an inhibition in the presence of the VPAC2 receptor antagonist PG 99-465, suggesting that activation of VPAC1 receptors inhibits and activation of VPAC2 receptors enhances, GABA release. A VPAC1 receptor agonist inhibited exocytotic voltage-gated calcium channel (VGCC)-dependent [3 H]-GABA release through activation of protein Gi/o , an effect also dependent on PKC activity. A VPAC2 receptor agonist enhanced both exocytotic VGCC-dependent release through protein Gs -dependent, PKA-dependent and PKC-dependent mechanisms and GABA transporter 1-mediated [3 H]-GABA release through a Gs protein-dependent and PKC-dependent mechanism.
Conclusions and implications: Our results show that VPAC1 and VPAC2 VIP receptors have opposing actions on GABA release from hippocampal nerve terminals through activation of different transduction pathways. As VPAC1 and VPAC2 receptors are located in different layers of Ammon's horn, our results suggest that these VIP receptors underlie different modulation of synaptic transmission to pyramidal cell dendrites and cell bodies, with important consequences for their possible therapeutic application in the treatment of epilepsy.
© 2017 The British Pharmacological Society.
Figures






Similar articles
-
VIP Modulation of Hippocampal Synaptic Plasticity: A Role for VIP Receptors as Therapeutic Targets in Cognitive Decline and Mesial Temporal Lobe Epilepsy.Front Cell Neurosci. 2020 Jun 12;14:153. doi: 10.3389/fncel.2020.00153. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32595454 Free PMC article. Review.
-
VIP enhances synaptic transmission to hippocampal CA1 pyramidal cells through activation of both VPAC1 and VPAC2 receptors.Brain Res. 2005 Jul 5;1049(1):52-60. doi: 10.1016/j.brainres.2005.04.077. Brain Res. 2005. PMID: 15935995
-
Endogenous inhibition of hippocampal LTD and depotentiation by vasoactive intestinal peptide VPAC1 receptors.Hippocampus. 2014 Nov;24(11):1353-63. doi: 10.1002/hipo.22316. Epub 2014 Jul 7. Hippocampus. 2014. PMID: 24935659
-
VPAC2 receptor activation mediates VIP enhancement of population spikes in the CA1 area of the hippocampus.Ann N Y Acad Sci. 2006 Jul;1070:210-4. doi: 10.1196/annals.1317.016. Ann N Y Acad Sci. 2006. PMID: 16888168
-
VPAC receptors: structure, molecular pharmacology and interaction with accessory proteins.Br J Pharmacol. 2012 May;166(1):42-50. doi: 10.1111/j.1476-5381.2011.01676.x. Br J Pharmacol. 2012. PMID: 21951273 Free PMC article. Review.
Cited by
-
VIP-Expressing GABAergic Neurons: Disinhibitory vs. Inhibitory Motif and Its Role in Communication Across Neocortical Areas.Front Cell Neurosci. 2022 Feb 10;16:811484. doi: 10.3389/fncel.2022.811484. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35221922 Free PMC article. Review.
-
Postweaning Development Influences Endogenous VPAC1 Modulation of LTP Induced by Theta-Burst Stimulation: A Link to Maturation of the Hippocampal GABAergic System.Biomolecules. 2024 Mar 20;14(3):379. doi: 10.3390/biom14030379. Biomolecules. 2024. PMID: 38540797 Free PMC article.
-
Vasoactive Intestinal Polypeptide in the Carotid Body-A History of Forty Years of Research. A Mini Review.Int J Mol Sci. 2020 Jun 30;21(13):4692. doi: 10.3390/ijms21134692. Int J Mol Sci. 2020. PMID: 32630153 Free PMC article. Review.
-
VIP Modulation of Hippocampal Synaptic Plasticity: A Role for VIP Receptors as Therapeutic Targets in Cognitive Decline and Mesial Temporal Lobe Epilepsy.Front Cell Neurosci. 2020 Jun 12;14:153. doi: 10.3389/fncel.2020.00153. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32595454 Free PMC article. Review.
-
VPAC1 and VPAC2 receptor deficiencies negatively influence pregnancy outcome through distinct and overlapping modulations of immune, trophoblast and vascular functions.Biochim Biophys Acta Mol Basis Dis. 2023 Feb;1869(2):166593. doi: 10.1016/j.bbadis.2022.166593. Epub 2022 Nov 1. Biochim Biophys Acta Mol Basis Dis. 2023. PMID: 36328148 Free PMC article.
References
-
- Acsády L, Arabadzisz D, Freund TF (1996a). Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide‐immunoreactive interneurons in rat hippocampus. Neuroscience 73: 299–315. - PubMed
-
- Acsády L, Görcs TJ, Freund TF (1996b). Different populations of vasoactive intestinal polypeptide‐immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience 73: 317–334. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

