Rapid colorimetric detection of Zika virus from serum and urine specimens by reverse transcription loop-mediated isothermal amplification (RT-LAMP)
- PMID: 28945787
- PMCID: PMC5612724
- DOI: 10.1371/journal.pone.0185340
Rapid colorimetric detection of Zika virus from serum and urine specimens by reverse transcription loop-mediated isothermal amplification (RT-LAMP)
Abstract
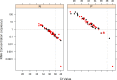
Zika virus (ZIKV) has emerged as a major global public health concern in the last two years due to its link as a causative agent of human birth defects. Its rapid expansion into the Western Hemisphere as well as the ability to be transmitted from mother to fetus, through sexual transmission and possibly through blood transfusions has increased the need for a rapid and expansive public health response to this unprecedented epidemic. A non-invasive and rapid ZIKV diagnostic screening assay that can be performed in a clinical setting throughout pregnancy is vital for prenatal care of women living in areas of the world where exposure to the virus is possible. To meet this need we have developed a sensitive and specific reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) assay to detect ZIKV RNA in urine and serum with a simple visual detection. RT-LAMP results were shown to have a limit of detection 10-fold higher than qRT-PCR. As little as 1.2 RNA copies/μl was detected by RT-LAMP from a panel of 178 diagnostic specimens. The assay was shown to be highly specific for ZIKV RNA when tested with diagnostic specimens positive for dengue virus (DENV) and chikungunya virus (CHIKV). The assay described here illustrates the potential for a fast, reliable, sensitive and specific assay for the detection of ZIKV from urine or serum that can be performed in a clinical or field setting with minimal equipment and technological expertise.
Conflict of interest statement
Figures

References
-
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–20. . - PubMed
-
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–9. doi: 10.3201/eid1408.080287 . - DOI - PMC - PubMed
-
- Campos GS, Bandeira AC, Sardi SI. Zika Virus Outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885–6. doi: 10.3201/eid2110.150847 . - DOI - PMC - PubMed
-
- Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110(4):569–72. doi: 10.1590/0074-02760150192 . - DOI - PMC - PubMed
-
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016;374(16):1552–63. doi: 10.1056/NEJMra1602113 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

