Human podocytes express functional thermosensitive TRPV channels
- PMID: 28945920
- PMCID: PMC5715976
- DOI: 10.1111/bph.14052
Human podocytes express functional thermosensitive TRPV channels
Abstract
Background and purpose: Heat-sensitive transient receptor potential vanilloid (TRPV) channels are expressed in various epithelial tissues regulating, among else, barrier functions. Their expression is well established in the distal nephron; however, we have no data about their presence in podocytes. As podocytes are indispensable in the formation of the glomerular filtration barrier, we investigated the presence and function of Ca2+ -permeable TRPV1-4 channels in human podocyte cultures.
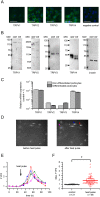
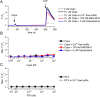
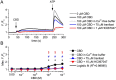
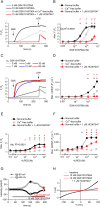
Experimental approach: Expression of TRPV1-4 channels was investigated at protein (immunocytochemistry, Western blot) and mRNA (Q-PCR) level in a conditionally immortalized human podocyte cell line. Channel function was assessed by measuring intracellular Ca2+ concentration using Flou-4 Ca2+ -indicator dye and patch clamp electrophysiology upon applying various activators and inhibitors.
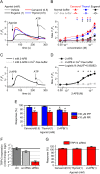
Key results: Thermosensitive TRP channels were expressed in podocytes. The TRPV1-specific agonists capsaicin and resiniferatoxin did not affect the intracellular Ca2+ concentration. Cannabidiol, an activator of TRPV2 and TRPV4 channels, induced moderate Ca2+ -influxes, inhibited by both tranilast and HC067047, blockers of TRPV2 and TRPV4 channels respectively. The TRPV4-specific agonists GSK1016790A and 4α-phorbol 12,13-didecanoate induced robust Ca2+ -signals which were abolished by HC067047. Non-specific agonists of TRPV3 channels induced marked Ca2+ transients. However, TRPV3 channel blockers, ruthenium red and isopentenyl diphosphate only partly inhibited the responses and TRPV3 silencing was ineffective suggesting remarkable off-target effects of the compounds.
Conclusion and implications: Our results indicate the functional presence of TRPV4 and other thermosensitive TRPV channels in human podocytes and raise the possibility of their involvement in the regulation of glomerular filtration barrier.
© 2017 The British Pharmacological Society.
Figures
Similar articles
-
Thermosensitive transient receptor potential channels in human corneal epithelial cells.J Cell Physiol. 2011 Jul;226(7):1828-42. doi: 10.1002/jcp.22514. J Cell Physiol. 2011. PMID: 21506114 Free PMC article.
-
Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation.Acta Physiol (Oxf). 2012 Feb;204(2):255-66. doi: 10.1111/j.1748-1716.2011.02338.x. Epub 2011 Aug 12. Acta Physiol (Oxf). 2012. PMID: 21726418
-
Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes.Am J Physiol Cell Physiol. 2006 Sep;291(3):C424-32. doi: 10.1152/ajpcell.00553.2005. Epub 2006 Apr 5. Am J Physiol Cell Physiol. 2006. PMID: 16597917
-
Renoprotection: focus on TRPV1, TRPV4, TRPC6 and TRPM2.Acta Physiol (Oxf). 2017 Mar;219(3):589-612. doi: 10.1111/apha.12828. Epub 2016 Dec 28. Acta Physiol (Oxf). 2017. PMID: 28028935 Review.
-
TRPV channels and vascular function.Acta Physiol (Oxf). 2011 Sep;203(1):99-116. doi: 10.1111/j.1748-1716.2010.02217.x. Epub 2010 Dec 9. Acta Physiol (Oxf). 2011. PMID: 21062421 Free PMC article. Review.
Cited by
-
Spice Up Your Kidney: A Review on the Effects of Capsaicin in Renal Physiology and Disease.Int J Mol Sci. 2024 Jan 8;25(2):791. doi: 10.3390/ijms25020791. Int J Mol Sci. 2024. PMID: 38255865 Free PMC article. Review.
-
Charting a TRP to Novel Therapeutic Destinations for Kidney Diseases.Trends Pharmacol Sci. 2019 Dec;40(12):911-918. doi: 10.1016/j.tips.2019.10.001. Epub 2019 Nov 5. Trends Pharmacol Sci. 2019. PMID: 31704171 Free PMC article. Review.
-
N,N-Dimethylglycine Sodium Salt Exerts Marked Anti-Inflammatory Effects in Various Dermatitis Models and Activates Human Epidermal Keratinocytes by Increasing Proliferation, Migration, and Growth Factor Release.Int J Mol Sci. 2023 Jul 9;24(14):11264. doi: 10.3390/ijms241411264. Int J Mol Sci. 2023. PMID: 37511024 Free PMC article.
-
Phagocytosis depends on TRPV2-mediated calcium influx and requires TRPV2 in lipids rafts: alteration in macrophages from patients with cystic fibrosis.Sci Rep. 2018 Mar 9;8(1):4310. doi: 10.1038/s41598-018-22558-5. Sci Rep. 2018. PMID: 29523858 Free PMC article.
-
Transient Receptor Potential Vanilloid in the Brain Gliovascular Unit: Prospective Targets in Therapy.Pharmaceutics. 2021 Mar 4;13(3):334. doi: 10.3390/pharmaceutics13030334. Pharmaceutics. 2021. PMID: 33806707 Free PMC article. Review.
References
-
- Akazawa Y, Yuki T, Yoshida H, Sugiyama Y, Inoue S (2013). Activation of TRPV4 strengthens the tight‐junction barrier in human epidermal keratinocytes. Skin Pharmacol Physiol 26: 15–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

