Macromolecular Antiviral Agents against Zika, Ebola, SARS, and Other Pathogenic Viruses
- PMID: 28945945
- PMCID: PMC7161897
- DOI: 10.1002/adhm.201700748
Macromolecular Antiviral Agents against Zika, Ebola, SARS, and Other Pathogenic Viruses
Abstract
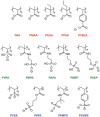
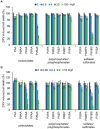
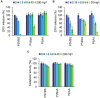
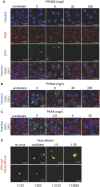
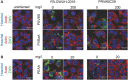
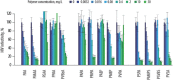
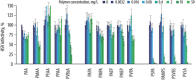
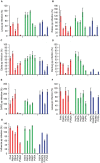
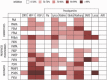
Viral pathogens continue to constitute a heavy burden on healthcare and socioeconomic systems. Efforts to create antiviral drugs repeatedly lag behind the advent of pathogens and growing understanding is that broad-spectrum antiviral agents will make strongest impact in future antiviral efforts. This work performs selection of synthetic polymers as novel broadly active agents and demonstrates activity of these polymers against Zika, Ebola, Lassa, Lyssa, Rabies, Marburg, Ebola, influenza, herpes simplex, and human immunodeficiency viruses. Results presented herein offer structure-activity relationships for these pathogens in terms of their susceptibility to inhibition by polymers, and for polymers in terms of their anionic charge and hydrophobicity that make up broad-spectrum antiviral agents. The identified leads cannot be predicted based on prior data on polymer-based antivirals and represent promising candidates for further development as preventive microbicides.
Keywords: Zika virus; broad spectrum antivirals; microbicides; polyanions; polymers.
© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Identification of a broad-spectrum antiviral small molecule against severe acute respiratory syndrome coronavirus and Ebola, Hendra, and Nipah viruses by using a novel high-throughput screening assay.J Virol. 2014 Apr;88(8):4353-65. doi: 10.1128/JVI.03050-13. Epub 2014 Feb 5. J Virol. 2014. PMID: 24501399 Free PMC article.
-
Arbidol and Other Low-Molecular-Weight Drugs That Inhibit Lassa and Ebola Viruses.J Virol. 2019 Apr 3;93(8):e02185-18. doi: 10.1128/JVI.02185-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30700611 Free PMC article.
-
Broad-Spectrum Antiviral Agents Based on Multivalent Inhibitors of Viral Infectivity.Adv Healthc Mater. 2021 Mar;10(6):e2001433. doi: 10.1002/adhm.202001433. Epub 2021 Jan 25. Adv Healthc Mater. 2021. PMID: 33491915 Free PMC article. Review.
-
Broad-Spectrum Antiviral Entry Inhibition by Interfacially Active Peptides.J Virol. 2020 Nov 9;94(23):e01682-20. doi: 10.1128/JVI.01682-20. Print 2020 Nov 9. J Virol. 2020. PMID: 32907984 Free PMC article.
-
Remdesivir against COVID-19 and Other Viral Diseases.Clin Microbiol Rev. 2020 Oct 14;34(1):e00162-20. doi: 10.1128/CMR.00162-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33055231 Free PMC article. Review.
Cited by
-
Mucus-Inspired Dynamic Hydrogels: Synthesis and Future Perspectives.J Am Chem Soc. 2022 Nov 9;144(44):20137-20152. doi: 10.1021/jacs.1c13547. Epub 2022 Sep 8. J Am Chem Soc. 2022. PMID: 36074739 Free PMC article. Review.
-
New Copolymers of Vinylphosphonic Acid with Hydrophilic Monomers and Their Eu3+ Complexes.Polymers (Basel). 2022 Jan 31;14(3):590. doi: 10.3390/polym14030590. Polymers (Basel). 2022. PMID: 35160579 Free PMC article.
-
Antibacterial and Antiviral Functional Materials: Chemistry and Biological Activity toward Tackling COVID-19-like Pandemics.ACS Pharmacol Transl Sci. 2020 Dec 29;4(1):8-54. doi: 10.1021/acsptsci.0c00174. eCollection 2021 Feb 12. ACS Pharmacol Transl Sci. 2020. PMID: 33615160 Free PMC article. Review.
-
Design of a New Vaccine Prototype against Porcine Circovirus Type 2 (PCV2), M. hyopneumoniae and M. hyorhinis Based on Multiple Antigens Microencapsulation with Sulfated Chitosan.Vaccines (Basel). 2024 May 17;12(5):550. doi: 10.3390/vaccines12050550. Vaccines (Basel). 2024. PMID: 38793801 Free PMC article.
-
Polymers Inspired by Heparin and Heparan Sulfate for Viral Targeting.Macromolecules. 2022 Sep 27;55(18):7957-7973. doi: 10.1021/acs.macromol.2c00675. Epub 2022 Sep 11. Macromolecules. 2022. PMID: 36186574 Free PMC article. Review.
References
-
- Burnouf T., Emmanuel J., Mbanya D., El‐Ekiaby M., Murphy W., Field S., Allain J. P., Lancet 2014, 384, 1347. - PubMed
-
- Afdhal N., Zeuzem S., Kwo P., Chojkier M., Gitlin N., Puoti M., Romero‐Gomez M., Zarski J. P., Agarwal K., Buggisch P., Foster G. R., Brau N., Buti M., Jacobson I. M., Subramanian G. M., Ding X., Mo H. M., Yang J. C., Pang P. S., Symonds W. T., McHutchison J. G., Muir A. J., Mangia A., Marcellin P., ION‐1 Investigators , N. Engl. J. Med. 2014, 370, 1889. - PubMed
-
- Feld J. J., Jacobson I. M., Hezode C., Asselah T., Ruane P. J., Gruener N., Abergel A., Mangia A., Lai C. L., Chan H. L. Y., Mazzotta F., Moreno C., Yoshida E., Shafran S. D., Towner W. J., Tran T. T., McNally J., Osinusi A., Svarovskaia E., Zhu Y., Brainard D. M., McHutchison J. G., Agarwal K., Zeuzem S., AON‐1 Investigators , N. Engl. J. Med. 2015, 373, 2599. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

