Apolipoprotein E4 inhibits autophagy gene products through direct, specific binding to CLEAR motifs
- PMID: 28945989
- PMCID: PMC6613789
- DOI: 10.1016/j.jalz.2017.07.754
Apolipoprotein E4 inhibits autophagy gene products through direct, specific binding to CLEAR motifs
Abstract
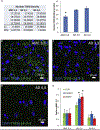
Introduction: Alzheimer apolipoprotein E (APOE) ɛ4/ɛ4 carriers have earlier disease onset and more protein aggregates than patients with other APOE genotypes. Autophagy opposes aggregation, and important autophagy genes are coordinately regulated by transcription factor EB (TFEB) binding to "coordinated lysosomal expression and regulation" (CLEAR) DNA motifs.
Methods: Autophagic gene expression was assessed in brains of controls and Alzheimer's disease (AD) patients parsed by APOE genotype and in a glioblastoma cell line expressing either apoE3 or apoE4. Computational modeling assessed interactions between apoE and mutated apoE with CLEAR or modified DNA.
Results: Three TFEB-regulated mRNA transcripts-SQSTM, MAP1LC3B, and LAMP2-were lower in AD ɛ4/ɛ4 than in AD ɛ3/ɛ3 brains. Computational modeling predicted avid specific binding of apoE4 to CLEAR motifs. ApoE was found in cellular nuclei, and in vitro binding assays suggest competition between apoE4 and TFEB at CLEAR sites.
Conclusion: ApoE4-CLEAR interactions may account for suppressed autophagy in APOE ɛ4/ɛ4 carriers and, in this way, contribute to earlier AD onset.
Keywords: APOE genotype; Alzheimer's disease; ApoE protein; DNA binding; EMSA; Molecular-dynamic simulation; PLA; Protein aggregation; TFEB; Transcription; autophagy.
Published by Elsevier Inc.
Figures



References
-
- Roses AD, Saunders AM, Alberts MA, Strittmatter WJ, Schmechel D, Gorder E, et al. Apolipoprotein E E4 allele and risk of dementia. Jama 1995;273:374–5. author reply 5–6. - PubMed
-
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet 2007;39:17–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

