Efficacy and safety of entecavir versus lamivudine over 5 years of treatment: A randomized controlled trial in Korean patients with hepatitis B e antigen-negative chronic hepatitis B
- PMID: 28946736
- PMCID: PMC5760004
- DOI: 10.3350/cmh.2016.0040
Efficacy and safety of entecavir versus lamivudine over 5 years of treatment: A randomized controlled trial in Korean patients with hepatitis B e antigen-negative chronic hepatitis B
Abstract
Background/aims: Long-term data on antiviral therapy in Korean patients with hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (CHB) are limited. This study evaluated the efficacy and safety of entecavir (ETV) and lamivudine (LAM) over 240 weeks.
Methods: Treatment-naive patients with HBeAg-negative CHB were randomized to receive ETV 0.5 mg/day or LAM 100 mg/day during the 96 week double-blind phase, followed by open-label treatment through week 240. The primary endpoint was the proportion of patients with virologic response (VR; hepatitis B virus [HBV] DNA<300 copies/mL) at week 24. Secondary objectives included alanine aminotransferase (ALT) normalization and emergence of ETV resistance (week 96), VR and log reduction in HBV DNA levels (week 240), and safety evaluation.
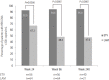
Results: In total, 120 patients (>16 years old) were included (ETV, n=56; LAM, n=64). Baseline characteristics were comparable between the two groups. A significantly higher proportion of ETV-treated patients achieved VR compared to LAM at week 24 (92.9% vs. 67.2%, P=0.0006), week 96 (94.6% vs. 48.4%, P<0.0001), and week 240 (95.0% vs. 47.6%, P<0.0001). At week 96, ALT normalization was observed in 87.5% and 51.6% of ETV and LAM patients, respectively (P<0.0001). Virologic breakthrough occurred in one patient (1.8%) receiving ETV and 26 patients (42.6%) receiving LAM (P<0.0001) up to week 96. Emergence of resistance to ETV was not detected. The incidence of serious adverse events was low and unrelated to the study medications.
Conclusions: Long-term ETV treatment was superior to LAM, with a significantly higher proportion of patients achieving VR. Both treatments were well tolerated.
Keywords: Entecavir; Hepatitis B; Lamivudine; Long-term effects.
Conflict of interest statement
Figures

Comment in
-
Lamivudine: fading into the mists of time.Clin Mol Hepatol. 2017 Dec;23(4):314-315. doi: 10.3350/cmh.2017.0110. Epub 2017 Nov 28. Clin Mol Hepatol. 2017. PMID: 29179530 Free PMC article. No abstract available.
References
-
- Chae HB, Kim JH, Kim JK, Yim HJ. Current status of liver diseases in Korea: hepatitis B. Korean J Hepatol. 2009;15 suppl 6:S13–S24. - PubMed
-
- Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen‐negative chronic hepatitis B. Hepatology. 2001;34:617–624. - PubMed
-
- Viganò M, Invernizzi F, Lampertico P. Optimal therapy of chronic hepatitis B: how do I treat my HBeAg-negative patients? Liver Int. 2015;35 Suppl 1:107–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

