Extracellular DNA and lipoteichoic acids interact with exopolysaccharides in the extracellular matrix of Streptococcus mutans biofilms
- PMID: 28946780
- PMCID: PMC5929139
- DOI: 10.1080/08927014.2017.1361412
Extracellular DNA and lipoteichoic acids interact with exopolysaccharides in the extracellular matrix of Streptococcus mutans biofilms
Abstract
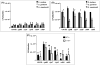
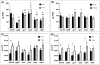
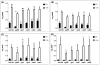
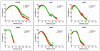
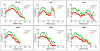
Streptococcus mutans-derived exopolysaccharides are virulence determinants in the matrix of biofilms that cause caries. Extracellular DNA (eDNA) and lipoteichoic acid (LTA) are found in cariogenic biofilms, but their functions are unclear. Therefore, strains of S. mutans carrying single deletions that would modulate matrix components were used: eDNA - ∆lytS and ∆lytT; LTA - ∆dltA and ∆dltD; and insoluble exopolysaccharide - ΔgtfB. Single-species (parental strain S. mutans UA159 or individual mutant strains) and mixed-species (UA159 or mutant strain, Actinomyces naeslundii and Streptococcus gordonii) biofilms were evaluated. Distinct amounts of matrix components were detected, depending on the inactivated gene. eDNA was found to be cooperative with exopolysaccharide in early phases, while LTA played a larger role in the later phases of biofilm development. The architecture of mutant strains biofilms was distinct (vs UA159), demonstrating that eDNA and LTA influence exopolysaccharide distribution and microcolony organization. Thus, eDNA and LTA may shape exopolysaccharide structure, affecting strategies for controlling pathogenic biofilms.
Keywords: Biofilms; Streptococcus mutans; eDNA; exopolysaccharides; extracellular matrix; lipoteichoic acids.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
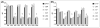




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
