Long-term outcome in survivors of neonatal tetanus following specialist intensive care in Vietnam
- PMID: 28946862
- PMCID: PMC5613471
- DOI: 10.1186/s12879-017-2748-3
Long-term outcome in survivors of neonatal tetanus following specialist intensive care in Vietnam
Abstract
Background: Neonatal tetanus continues to occur in many resource-limited settings but there are few data regarding long-term neurological outcome from the disease, especially in settings with critical care facilities.
Methods: We assessed long-term outcome following neonatal tetanus in infants treated in a pediatric intensive care unit in southern Vietnam. Neurological and neurodevelopmental testing was performed in 17 survivors of neonatal tetanus and 18 control children from the same communities using tools previously validated in Vietnamese children.
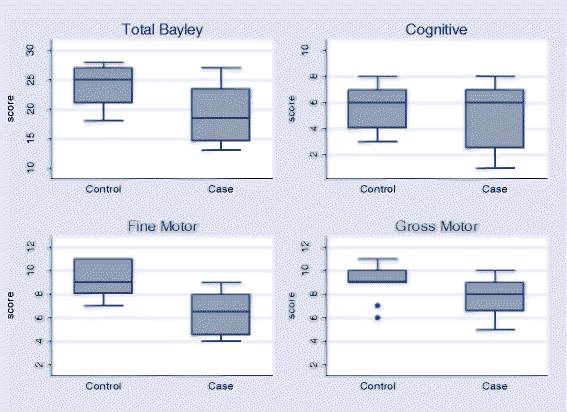
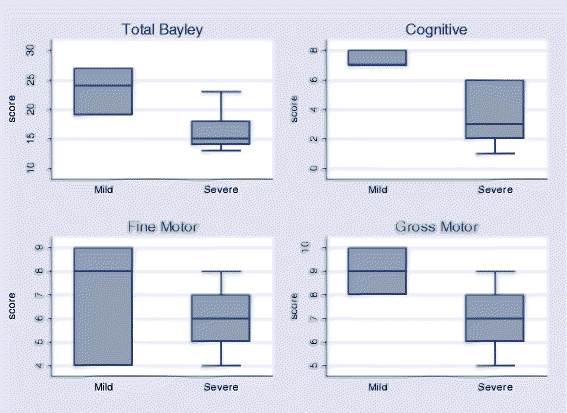
Results: The median age of children assessed was 36 months. Eight neonatal tetanus survivors and 9 community control cases aged < 42 months were tested using the Bayley III Scales of Infant and Toddler Development (Bayley III-VN) and 8 neonatal tetanus survivors and 9 community controls aged ≥42 months were tested using the Movement Assessment Battery for Children. No significant reductions in growth indices or neurodevelopmental scores were shown in survivors of neonatal tetanus compared to controls although there was a trend towards lower scores in neonatal tetanus survivors. Neurological examination was normal in all children except for two neonatal tetanus survivors with perceptive deafness and one child with mild gross motor abnormality. Neonatal tetanus survivors who had expienced severe disease (Ablett grade ≥ 3) had lower total Bayley III-VN scores than those with mild disease (15 (IQR 14-18) vs 24 (IQR 19-27), p = 0.05) with a significantly lower cognitive domain score (3 (IQR 2-6) severe disease vs 7 (IQR 7-8) mild disease, p = 0.02).
Conclusions: Neonatal tetanus is associated with long-term sequelae in those with severe disease. In view of these findings, prevention of neonatal tetanus should remain a priority.
Keywords: Development; Neonatal tetanus; Outcome.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Hospital for Tropical Diseases Ethics Committee (Number CS/ND/15/28). Parents of participants signed informed consent forms before participating in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Jeena PM, Coovadia HM, Gouws E. Risk factors for neonatal tetanus in KwaZulu-Natal. S Afr Med J. 1997;87(1):46–48. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

