Autonomous and non-autonomous roles for ephrin-B in interneuron migration
- PMID: 28947178
- PMCID: PMC5658245
- DOI: 10.1016/j.ydbio.2017.09.024
Autonomous and non-autonomous roles for ephrin-B in interneuron migration
Abstract
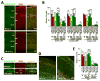
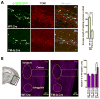
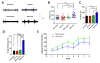
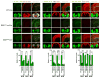
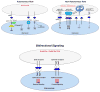
While several studies indicate the importance of ephrin-B/EphB bidirectional signaling in excitatory neurons, potential roles for these molecules in inhibitory neurons are largely unknown. We identify here an autonomous receptor-like role for ephrin-B reverse signaling in the tangential migration of interneurons into the neocortex using ephrin-B (EfnB1/B2/B3) conditional triple mutant (TMlz) mice and a forebrain inhibitory neuron specific Cre driver. Inhibitory neuron deletion of the three EfnB genes leads to reduced interneuron migration, abnormal cortical excitability, and lethal audiogenic seizures. Truncated and intracellular point mutations confirm the importance of ephrin-B reverse signaling in interneuron migration and cortical excitability. A non-autonomous ligand-like role was also identified for ephrin-B2 that is expressed in neocortical radial glial cells and required for proper tangential migration of GAD65-positive interneurons. Our studies thus define both receptor-like and ligand-like roles for the ephrin-B molecules in controlling the migration of interneurons as they populate the neocortex and help establish excitatory/inhibitory (E/I) homeostasis.
Keywords: Bidirectional signaling; EphB; Ephrin-B; Excitatory/inhibitory homeostasis; Inhibitory interneuron migration.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

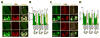

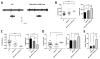

Similar articles
-
Abnormalities in cortical interneuron subtypes in ephrin-B mutant mice.Eur J Neurosci. 2018 Jul;48(2):1803-1817. doi: 10.1111/ejn.14022. Epub 2018 Jul 10. Eur J Neurosci. 2018. PMID: 29904965 Free PMC article.
-
Ephrin-A2 regulates excitatory neuron differentiation and interneuron migration in the developing neocortex.Sci Rep. 2017 Sep 18;7(1):11813. doi: 10.1038/s41598-017-12185-x. Sci Rep. 2017. PMID: 28924206 Free PMC article.
-
The protocadherin gene Celsr3 is required for interneuron migration in the mouse forebrain.Mol Cell Biol. 2009 Jun;29(11):3045-61. doi: 10.1128/MCB.00011-09. Epub 2009 Mar 30. Mol Cell Biol. 2009. PMID: 19332558 Free PMC article.
-
Migratory pathways of GABAergic interneurons when they enter the neocortex.Eur J Neurosci. 2012 Jun;35(11):1655-60. doi: 10.1111/j.1460-9568.2012.08111.x. Epub 2012 May 28. Eur J Neurosci. 2012. PMID: 22639844 Review.
-
Cellular and molecular mechanisms controlling the migration of neocortical interneurons.Eur J Neurosci. 2013 Jul;38(1):2019-29. doi: 10.1111/ejn.12225. Epub 2013 May 8. Eur J Neurosci. 2013. PMID: 23651101 Review.
Cited by
-
EphrinB2-mediated chondrocyte autophagy induces post-traumatic arthritis via rupture of cartilage homeostasis.J Cell Mol Med. 2024 Sep;28(18):e70095. doi: 10.1111/jcmm.70095. J Cell Mol Med. 2024. PMID: 39289794 Free PMC article.
-
JNK signaling is required for proper tangential migration and laminar allocation of cortical interneurons.Development. 2020 Jan 17;147(2):dev180646. doi: 10.1242/dev.180646. Development. 2020. PMID: 31915148 Free PMC article.
-
EphB2 Signaling Is Implicated in Astrocyte-Mediated Parvalbumin Inhibitory Synapse Development.J Neurosci. 2024 Nov 6;44(45):e0154242024. doi: 10.1523/JNEUROSCI.0154-24.2024. J Neurosci. 2024. PMID: 39327008 Free PMC article.
-
Functional Consequences of Synapse Remodeling Following Astrocyte-Specific Regulation of Ephrin-B1 in the Adult Hippocampus.J Neurosci. 2018 Jun 20;38(25):5710-5726. doi: 10.1523/JNEUROSCI.3618-17.2018. Epub 2018 May 23. J Neurosci. 2018. PMID: 29793972 Free PMC article.
-
DNMT1-mediated regulation of somatostatin-positive interneuron migration impacts cortical architecture and function.Nat Commun. 2025 Jul 24;16(1):6834. doi: 10.1038/s41467-025-62114-0. Nat Commun. 2025. PMID: 40707493 Free PMC article.
References
-
- Andrews W, Barber M, Hernadez-Miranda LR, Xian J, Rakic S, Sundaresan V, Rabbitts TH, Pannell R, Rabbitts P, Thompson H, Erskine L, Murakami F, Parnavelas JG. The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons. Dev Biol. 2008;313:648–658. doi: 10.1016/j.ydbio.2007.10.052. - DOI - PubMed
-
- Bennett KM, Afanador MD, Lal CV, Xu H, Persad E, Legan SK, Chenaux G, Dellinger M, Savani RC, Dravis C, Henkemeyer M, Schwarz MA. Ephrin-B2 reverse signaling increases alpha5beta1 integrin-mediated fibronectin deposition and reduces distal lung compliance. Am J Respir Cell Mol Biol. 2013;49:680–687. doi: 10.1165/rcmb.2013-0002OC. - DOI - PMC - PubMed
-
- Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103:1043–1050. S0306452201000367 [pii] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

