Study protocol for a randomised controlled trial evaluating the effect of prenatal omega-3 LCPUFA supplementation to reduce the incidence of preterm birth: the ORIP trial
- PMID: 28947468
- PMCID: PMC5623491
- DOI: 10.1136/bmjopen-2017-018360
Study protocol for a randomised controlled trial evaluating the effect of prenatal omega-3 LCPUFA supplementation to reduce the incidence of preterm birth: the ORIP trial
Abstract
Introduction: Preterm birth accounts for more than 85% of all perinatal complications and deaths. Seventy-five per cent of early preterm births (EPTBs) occur spontaneously and without identifiable risk factors. The need for a broadly applicable, effective strategy for primary prevention is paramount. Secondary outcomes from the docosahexaenoic acid (DHA) to Optimise Mother Infant Outcome trial showed that maternal supplementation until delivery with omega-3 (ω-3) long chain polyunsaturated fatty acid (LCPUFA), predominantly as DHA, resulted in a 50% reduction in the incidence of EPTB and an increase in the incidence of post-term induction or post-term prelabour caesarean section due to extended gestation. We aim to determine the effectiveness of supplementing the maternal diet with ω-3 LCPUFA until 34 weeks' gestation on the incidence of EPTB.
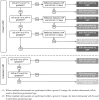
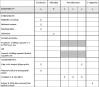
Methods and analysis: This is a multicentre, parallel group, randomised, blinded and controlled trial. Women less than 20 weeks' gestation with a singleton or multiple pregnancy and able to give informed consent are eligible to participate. Women will be randomised to receive high DHA fish oil capsules or control capsules without DHA. Capsules will be taken from enrolment until 34 weeks' gestation. The primary outcome is the incidence of EPTB, defined as delivery before 34 completed weeks' gestation. Key secondary outcomes include length of gestation, incidence of post-term induction or prelabour caesarean section and spontaneous EPTB. The target sample size is 5540 women (2770 per group), which will provide 85% power to detect an absolute reduction in the incidence of preterm birth of 1.16% (from 2.45% to 1.29%) between the DHA and control group (two sided α=0.05). The primary analysis will be based on the intention-to-treat principle.
Trial registration number: Australia and New Zealand Clinical Trial Registry Number: 2613001142729; Pre-results.
Keywords: maternal diet; ocosahexaenoic acid; omega-3 long chain polyunsaturated fatty acids; pregnancy; preterm birth; preventive medicine.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: The authors declare: financial support for the submitted work from the National Health and Medical Research Council (NHMRC) Australia (Project Grant 1050468). RG serves on a scientific advisory board for Fonterra; MM serves on scientific advisory boards for Nestle and Fonterra. Associated Honoraria for RG and MM are paid to their institutions to support conference travel and continuing education for postgraduate students and early career researchers. The authors declare that they have no competing interests.
Figures
References
-
- Thornton S. Preterm Birth: Causes, Consequences and Prevention. The Obstetrician & Gynaecologist 2008;10:280–80. 10.1576/toag.10.4.280b.27453 - DOI
-
- Lumley J. Defining the problem: the epidemiology of preterm birth. BJOG 2003;110 (Suppl 20):3–7. - PubMed
-
- Martin JA, Hamilton BE, Sutton PD, et al. . Births: final data for 2004. Natl Vital Stat Rep 2006;55:1–101. - PubMed
-
- Gravett MG. Causes of preterm delivery. Semin Perinatol 1984;8:246–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
