Genetics of Nevirapine Metabolic Pathways at Steady State in HIV-Infected Cambodians
- PMID: 28947469
- PMCID: PMC5700353
- DOI: 10.1128/AAC.00733-17
Genetics of Nevirapine Metabolic Pathways at Steady State in HIV-Infected Cambodians
Abstract
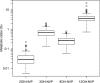
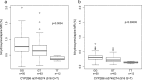
Nevirapine is metabolized by several hepatic cytochrome P450 (CYP) isoforms to generate four primary hydroxylated metabolites: 2-hydroxynevirapine, 3-hydroxynevirapine, 8-hydroxynevirapine, and 12-hydroxynevirapine. The present study characterized associations between genetic polymorphisms and metabolite ratios in HIV-infected Cambodians. We demonstrate associations between CYP2B6 polymorphisms and metabolite ratios for both 3-hydroxynevirapine and 8-hydroxynevirapine, suggesting involvement of CYP2B6 in generating these metabolites.
Keywords: genetic polymorphisms; metabolism pathways; nevirapine; population pharmacokinetics.
Copyright © 2017 American Society for Microbiology.
Figures
References
-
- World Health Organization. 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2nd ed World Health Organization, Geneva, Switzerland. - PubMed
-
- Riska P, Lamson M, MacGregor T, Sabo J, Hattox S, Pav J, Keirns J. 1999. Disposition and biotransformation of the antiretroviral drug nevirapine in humans. Drug Metab Dispos 27:895–901. - PubMed
-
- Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, Keiser O, Biollaz J, Décosterd L, Telenti A, Swiss HIV Cohort Study. 2005. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics 15:1–5. doi:10.1097/01213011-200501000-00001. - DOI - PubMed
-
- Wyen C, Hendra H, Vogel M, Hoffmann C, Knechten H, Brockmeyer NH, Bogner JR, Rockstroh J, Esser S, Jaeger H, Harrer T, Mauss S, van Lunzen J, Skoetz N, Jetter A, Groneuer C, Fatkenheuer G, Khoo SH, Egan D, Back DJ, Owen A, German Competence Network for HIV/ AIDS. 2008. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother 61:914–918. doi:10.1093/jac/dkn029. - DOI - PMC - PubMed
-
- Chou M, Bertrand J, Segeral O, Verstuyft C, Borand L, Comets E, Le Tiec C, Becquemont L, Ouk V, Mentre F, Taburet A-M. 2010. Population pharmacokinetic-pharmacogenetic study of nevirapine in HIV-infected Cambodian patients. Antimicrob Agents Chemother 54:4432–4439. doi:10.1128/AAC.00512-10. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

