Contribution of Streptococcus mutans Strains with Collagen-Binding Proteins in the Presence of Serum to the Pathogenesis of Infective Endocarditis
- PMID: 28947650
- PMCID: PMC5695098
- DOI: 10.1128/IAI.00401-17
Contribution of Streptococcus mutans Strains with Collagen-Binding Proteins in the Presence of Serum to the Pathogenesis of Infective Endocarditis
Abstract
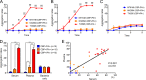
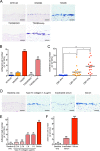
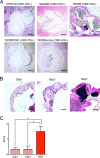
Streptococcus mutans, a major pathogen of dental caries, is considered one of the causative agents of infective endocarditis (IE). Recently, bacterial DNA encoding 120-kDa cell surface collagen-binding proteins (CBPs) has frequently been detected from S. mutans-positive IE patients. In addition, some of the CBP-positive S. mutans strains lacked a 190-kDa protein antigen (PA), whose absence strengthened the adhesion to and invasion of endothelial cells. The interaction between pathogenic bacteria and serum or plasma is considered an important virulence factor in developing systemic diseases; thus, we decided to analyze the pathogenesis of IE induced by S. mutans strains with different patterns of CBP and PA expression by focusing on the interaction with serum or plasma. CBP-positive (CBP+)/PA-negative (PA-) strains showed prominent aggregation in the presence of human serum or plasma, which was significantly greater than that with CBP+/PA-positive (PA+) and CBP-negative (CBP-)/PA+ strains. Aggregation of CBP+/PA- strains was also observed in the presence of a high concentration of type IV collagen, a major extracellular matrix protein in serum. In addition, aggregation of CBP+/PA- strains was drastically reduced when serum complement was inactivated. Furthermore, an ex vivo adherence model and an in vivo rat model of IE showed that extirpated heart valves infected with CBP+/PA- strains displayed prominent bacterial mass formation, which was not observed following infection with CBP+/PA+ and CBP-/PA+ strains. These results suggest that CBP+/PA-S. mutans strains utilize serum to contribute to their pathogenicity in IE.
Keywords: Streptococcus mutans; blood; infective endocarditis.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
Potential high virulence for infective endocarditis in Streptococcus mutans strains with collagen-binding proteins but lacking PA expression.Arch Oral Biol. 2013 Nov;58(11):1627-34. doi: 10.1016/j.archoralbio.2013.06.008. Epub 2013 Sep 10. Arch Oral Biol. 2013. PMID: 24112728
-
Contribution of the interaction of Streptococcus mutans serotype k strains with fibrinogen to the pathogenicity of infective endocarditis.Infect Immun. 2014 Dec;82(12):5223-34. doi: 10.1128/IAI.02164-14. Epub 2014 Oct 6. Infect Immun. 2014. PMID: 25287921 Free PMC article.
-
Contribution of the Collagen-Binding Proteins of Streptococcus mutans to Bacterial Colonization of Inflamed Dental Pulp.PLoS One. 2016 Jul 21;11(7):e0159613. doi: 10.1371/journal.pone.0159613. eCollection 2016. PLoS One. 2016. PMID: 27442266 Free PMC article.
-
From Teeth to Body: The Complex Role of Streptococcus mutans in Systemic Diseases.Mol Oral Microbiol. 2025 Apr;40(2):65-81. doi: 10.1111/omi.12491. Epub 2025 Jan 27. Mol Oral Microbiol. 2025. PMID: 39865888 Review.
-
Roles of oral bacteria in cardiovascular diseases--from molecular mechanisms to clinical cases: Cell-surface structures of novel serotype k Streptococcus mutans strains and their correlation to virulence.J Pharmacol Sci. 2010;113(2):120-5. doi: 10.1254/jphs.09r24fm. Epub 2010 May 24. J Pharmacol Sci. 2010. PMID: 20501965 Review.
Cited by
-
Edwardsiella piscicida employs C3 derivative to mediate internalization and infection via the teleost-specific receptor.Virulence. 2025 Dec;16(1):2526783. doi: 10.1080/21505594.2025.2526783. Epub 2025 Jul 3. Virulence. 2025. PMID: 40583841 Free PMC article.
-
Infective Endocarditis Following Coil Embolization for a Visceral Pseudoaneurysm: A Case Report.Cureus. 2025 Aug 6;17(8):e89484. doi: 10.7759/cureus.89484. eCollection 2025 Aug. Cureus. 2025. PMID: 40777070 Free PMC article.
-
Evaluation of the collagen-binding properties and virulence of killed Streptococcus mutans in a silkworm model.Sci Rep. 2022 Feb 18;12(1):2800. doi: 10.1038/s41598-022-06345-x. Sci Rep. 2022. PMID: 35181690 Free PMC article.
-
Potential involvement of Streptococcus mutans possessing collagen binding protein Cnm in infective endocarditis.Sci Rep. 2020 Nov 5;10(1):19118. doi: 10.1038/s41598-020-75933-6. Sci Rep. 2020. PMID: 33154489 Free PMC article.
-
New insights into iron uptake in Streptococcus mutans: evidence for a role of siderophore-like molecules.Arch Microbiol. 2025 Mar 20;207(4):96. doi: 10.1007/s00203-025-04284-5. Arch Microbiol. 2025. PMID: 40111578
References
-
- Nakatani S, Mitsutake K, Hozumi T, Yoshikawa J, Akiyama M, Yoshida K, Ishizuka N, Nakamura K, Taniguchi Y, Yoshioka K, Kawazoe K, Akaishi M, Niwa K, Nakazawa M, Kitamura S, Miyatake K. 2003. Committee on Guideline for Prevention and Management of Infective Endocarditis, Japanese Circulation Society. Current characteristics of infective endocarditis in Japan: an analysis of 848 cases in 2000 and 2001. Circ J 67:901–905. - PubMed
-
- Roberts GJ, Lucas VS, Omar J. 2000. Bacterial endocarditis and orthodontics. J R Coll Surg Edinb 45:141–145. - PubMed
-
- Simon GL. 1984. Transient bacteremia and endocarditis prophylaxis. Arch Intern Med 144:34–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

