A metabolic pathway for catabolizing levulinic acid in bacteria
- PMID: 28947739
- PMCID: PMC5705400
- DOI: 10.1038/s41564-017-0028-z
A metabolic pathway for catabolizing levulinic acid in bacteria
Abstract
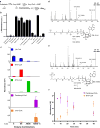
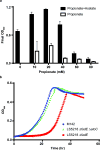
Microorganisms can catabolize a wide range of organic compounds and therefore have the potential to perform many industrially relevant bioconversions. One barrier to realizing the potential of biorefining strategies lies in our incomplete knowledge of metabolic pathways, including those that can be used to assimilate naturally abundant or easily generated feedstocks. For instance, levulinic acid (LA) is a carbon source that is readily obtainable as a dehydration product of lignocellulosic biomass and can serve as the sole carbon source for some bacteria. Yet, the genetics and structure of LA catabolism have remained unknown. Here, we report the identification and characterization of a seven-gene operon that enables LA catabolism in Pseudomonas putida KT2440. When the pathway was reconstituted with purified proteins, we observed the formation of four acyl-CoA intermediates, including a unique 4-phosphovaleryl-CoA and the previously observed 3-hydroxyvaleryl-CoA product. Using adaptive evolution, we obtained a mutant of Escherichia coli LS5218 with functional deletions of fadE and atoC that was capable of robust growth on LA when it expressed the five enzymes from the P. putida operon. This discovery will enable more efficient use of biomass hydrolysates and metabolic engineering to develop bioconversions using LA as a feedstock.
Figures

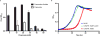
Comment in
-
Pathway towards renewable chemicals.Nat Microbiol. 2017 Dec;2(12):1580-1581. doi: 10.1038/s41564-017-0071-9. Nat Microbiol. 2017. PMID: 29176700 No abstract available.
Similar articles
-
Growth-coupled bioconversion of levulinic acid to butanone.Metab Eng. 2019 Sep;55:92-101. doi: 10.1016/j.ymben.2019.06.003. Epub 2019 Jun 19. Metab Eng. 2019. PMID: 31226347 Free PMC article.
-
Engineering the lva operon and Optimization of Culture Conditions for Enhanced Production of 4-Hydroxyvalerate from Levulinic Acid in Pseudomonas putida KT2440.J Agric Food Chem. 2019 Mar 6;67(9):2540-2546. doi: 10.1021/acs.jafc.8b06884. Epub 2019 Feb 22. J Agric Food Chem. 2019. PMID: 30773878
-
Microbial production of levulinic acid from glucose by engineered Pseudomonas putida KT2440.J Biotechnol. 2024 Nov 20;395:161-169. doi: 10.1016/j.jbiotec.2024.09.015. Epub 2024 Sep 28. J Biotechnol. 2024. PMID: 39343057
-
Loving the poison: the methylcitrate cycle and bacterial pathogenesis.Microbiology (Reading). 2018 Mar;164(3):251-259. doi: 10.1099/mic.0.000604. Epub 2018 Jan 22. Microbiology (Reading). 2018. PMID: 29458664 Review.
-
Biotechnological production of aromatic compounds of the extended shikimate pathway from renewable biomass.J Biotechnol. 2017 Sep 10;257:211-221. doi: 10.1016/j.jbiotec.2016.11.016. Epub 2016 Nov 18. J Biotechnol. 2017. PMID: 27871872 Review.
Cited by
-
GapMind: Automated Annotation of Amino Acid Biosynthesis.mSystems. 2020 Jun 23;5(3):e00291-20. doi: 10.1128/mSystems.00291-20. mSystems. 2020. PMID: 32576650 Free PMC article.
-
Experimental and Analytical Approaches for Improving the Resolution of Randomly Barcoded Transposon Insertion Sequencing (RB-TnSeq) Studies.ACS Synth Biol. 2022 Jun 17;11(6):2015-2021. doi: 10.1021/acssynbio.2c00119. Epub 2022 Jun 3. ACS Synth Biol. 2022. PMID: 35657709 Free PMC article.
-
Genome-wide fitness profiling reveals molecular mechanisms that bacteria use to interact with Trichoderma atroviride exometabolites.PLoS Genet. 2023 Aug 31;19(8):e1010909. doi: 10.1371/journal.pgen.1010909. eCollection 2023 Aug. PLoS Genet. 2023. PMID: 37651474 Free PMC article.
-
Genetic tools for reliable gene expression and recombineering in Pseudomonas putida.J Ind Microbiol Biotechnol. 2018 Jul;45(7):517-527. doi: 10.1007/s10295-017-2001-5. Epub 2018 Jan 3. J Ind Microbiol Biotechnol. 2018. PMID: 29299733 Free PMC article.
-
Need for Laboratory Ecosystems To Unravel the Structures and Functions of Soil Microbial Communities Mediated by Chemistry.mBio. 2018 Jul 17;9(4):e01175-18. doi: 10.1128/mBio.01175-18. mBio. 2018. PMID: 30018110 Free PMC article.
References
-
- Pileidis FD, Titirici MM. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem. 2016;9:562–582. - PubMed
-
- Bozell JJ, et al. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000;28:227–239.
-
- Werpy T, Petersen G. Top Value Added Chemicals from Biomass. Program. 2004;76 doi: 10.2172/926125. - DOI
-
- Lange JP, et al. Valeric biofuels: A platform of cellulosic transportation fuels. Angew. Chemie - Int. Ed. 2010;49:4479–4483. - PubMed
-
- Alonso DM, Wettstein SG, Dumesic JA. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013;15:584.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

