Human Cerebrospinal fluid promotes long-term neuronal viability and network function in human neocortical organotypic brain slice cultures
- PMID: 28947761
- PMCID: PMC5613008
- DOI: 10.1038/s41598-017-12527-9
Human Cerebrospinal fluid promotes long-term neuronal viability and network function in human neocortical organotypic brain slice cultures
Abstract
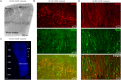
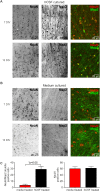
Pathophysiological investigation of CNS-related diseases, such as epilepsy or neurodegenerative disorders, largely relies on histological studies on human post mortem tissue, tissue obtained by biopsy or resective surgery and on studies using disease models including animal models, heterologous expression systems or cell culture based approaches. However, in general it remains elusive to what extent results obtained in model systems can be directly translated to the human brain, calling for strategies allowing validation or even primary investigation in live human CNS tissue. In the work reported here, we prepared human organotypic slice cultures from access tissue of resective epilepsy surgery. Employing different culture conditions, we systematically compared artificial culturing media versus human cerbrospinal fluid (hCSF) obtained from patients with normal pressure hydrocephalus (NPH). Presented data demonstrates sustained cortical neuronal survival including not only maintenance of typical cellular electrophysiological properties and activity, such as robust action potential generation and synaptic connectivity, but also preservation of tonic and phasic network activity up to several weeks in vitro. As clearly delineated by immunocytochemistry, single cell patch clamp and extracellular recordings, we find that in contrast to artificial culturing media, hCSF significantly enhances neuron viability and maintenance of network activity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

