Stereoselective one-pot synthesis of polypropionates
- PMID: 28947767
- PMCID: PMC5612996
- DOI: 10.1038/s41467-017-00787-y
Stereoselective one-pot synthesis of polypropionates
Abstract
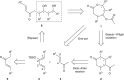
Polypropionates-motifs with alternating methyl and hydroxy groups-are important segments of many natural products possessing high bioactivity and therapeutic value. Synthetic access to these structures remains an area of intensive interest, focusing on the establishment of the contiguous stereocentres and a desire for operational simplicity. Here we report an efficient strategy for the stereoselective assembly of polypropionates with three or four stereocentres through a three-step relay process that include Diels-Alder reaction, silylenol ether hydrolysis and Baeyer-Villiger oxidation. The stereochemistry and functionality of the resulting polypropionates depend on the substitution pattern of the diene and dienophile substrates of the Diels-Alder cycloaddition. More importantly, the relay sequence is effectively performed in one pot, and the product could potentially undergo the same sequence for further elaboration. Finally, the C1-C9 segment of the macrolide etnangien is constructed with four of the six stereogenic centres established using the relay sequence.Polypropionates are present in many natural products possessing high bioactivity and therapeutic value. Here the authors show a strategy for the stereoselective assembly of polypropionates with three or four stereocentres through a process that includes a Diels-Alder reaction, silylenol ether hydrolysis and Baeyer-Villiger oxidation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Remote Asymmetric Induction Reactions using a E, E-Vinylketene Silyl N, O-Acetal and the Wide Range Stereocontrol Strategy for the Synthesis of Polypropionates.Acc Chem Res. 2018 May 15;51(5):1301-1314. doi: 10.1021/acs.accounts.8b00125. Epub 2018 May 4. Acc Chem Res. 2018. PMID: 29726677
-
Stereoselective synthesis of the spirocyclic core of 13-desmethyl spirolide C using an aza-Claisen rearrangement and an exo-selective Diels-Alder cycloaddition.Org Biomol Chem. 2023 Feb 8;21(6):1222-1234. doi: 10.1039/d2ob01992b. Org Biomol Chem. 2023. PMID: 36633001
-
Multicomponent Hetero-[4 + 2] Cycloaddition/Allylboration Reaction: From Natural Product Synthesis to Drug Discovery.Acc Chem Res. 2016 Nov 15;49(11):2489-2500. doi: 10.1021/acs.accounts.6b00403. Epub 2016 Oct 18. Acc Chem Res. 2016. PMID: 27753496
-
Progress in Lewis-Acid-Templated Diels-Alder Reactions.Molecules. 2024 Mar 6;29(5):1187. doi: 10.3390/molecules29051187. Molecules. 2024. PMID: 38474699 Free PMC article. Review.
-
Application of the aza-Diels-Alder reaction in the synthesis of natural products.Org Biomol Chem. 2017 Apr 11;15(15):3105-3129. doi: 10.1039/c6ob02761j. Org Biomol Chem. 2017. PMID: 28327756 Review.
Cited by
-
Enantioselective Total Synthesis of (+)-Heilonine.J Am Chem Soc. 2021 Oct 13;143(40):16394-16400. doi: 10.1021/jacs.1c08756. Epub 2021 Sep 29. J Am Chem Soc. 2021. PMID: 34585920 Free PMC article.
-
Synthesis of highly effective polyester/polyacrylate compatibilizers using switchable polymerization.Nat Commun. 2025 Mar 4;16(1):2154. doi: 10.1038/s41467-025-57449-7. Nat Commun. 2025. PMID: 40038273 Free PMC article.
References
-
- Rimando, A. M. & Baerson, S. R. Polyketides: Biosynthesis, Biological Activity, and Genetic Engineering (American Chemical Society, 2007).
-
- Longley, R. E. in Natural Products and Cancer Drug Discovery (ed. Koehn, F. E.) Ch. 3, 39−58 (Springer, 2013).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

