Apolipoprotein E epsilon 4 (APOE- ε 4) genotype is associated with decreased 6-month verbal memory performance after mild traumatic brain injury
- PMID: 28948085
- PMCID: PMC5607554
- DOI: 10.1002/brb3.791
Apolipoprotein E epsilon 4 (APOE- ε 4) genotype is associated with decreased 6-month verbal memory performance after mild traumatic brain injury
Abstract
Introduction: The apolipoprotein E (APOE) ε4 allele associates with memory impairment in neurodegenerative diseases. Its association with memory after mild traumatic brain injury (mTBI) is unclear.
Methods: mTBI patients (Glasgow Coma Scale score 13-15, no neurosurgical intervention, extracranial Abbreviated Injury Scale score ≤1) aged ≥18 years with APOE genotyping results were extracted from the Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot (TRACK-TBI Pilot) study. Cohorts determined by APOE-ε4(+/-) were assessed for associations with 6-month verbal memory, measured by California Verbal Learning Test, Second Edition (CVLT-II) subscales: Immediate Recall Trials 1-5 (IRT), Short-Delay Free Recall (SDFR), Short-Delay Cued Recall (SDCR), Long-Delay Free Recall (LDFR), and Long-Delay Cued Recall (LDCR). Multivariable regression controlled for demographic factors, seizure history, loss of consciousness, posttraumatic amnesia, and acute intracranial pathology on computed tomography (CT).
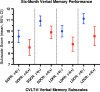
Results: In 114 mTBI patients (APOE-ε4(-)=79; APOE-ε4(+)=35), ApoE-ε4(+) was associated with long-delay verbal memory deficits (LDFR: B = -1.17 points, 95% CI [-2.33, -0.01], p = .049; LDCR: B = -1.58 [-2.63, -0.52], p = .004), and a marginal decrease on SDCR (B = -1.02 [-2.05, 0.00], p = .050). CT pathology was the strongest predictor of decreased verbal memory (IRT: B = -8.49, SDFR: B = -2.50, SDCR: B = -1.85, LDFR: B = -2.61, LDCR: B = -2.60; p < .001). Seizure history was associated with decreased short-term memory (SDFR: B = -1.32, p = .037; SDCR: B = -1.44, p = .038).
Conclusion: The APOE-ε4 allele may confer an increased risk of impairment of 6-month verbal memory for patients suffering mTBI, with implications for heightened surveillance and targeted therapies. Acute intracranial pathology remains the driver of decreased verbal memory performance at 6 months after mTBI.
Keywords: apolipoprotein E; genetic factors; human studies; outcome measures; traumatic brain injury; verbal memory.
Figures
References
-
- Alexander, S. , Kerr, M. E. , Kim, Y. , Kamboh, M. I. , Beers, S. R. , & Conley, Y. P. (2007). Apolipoprotein E4 allele presence and functional outcome after severe traumatic brain injury. Journal of Neurotrauma, 24, 790–797. - PubMed
-
- Anderson, G. D. , Temkin, N. R. , Dikmen, S. S. , Diaz‐Arrastia, R. , Machamer, J. E. , Farhrenbruch, C. , … Sadrzadeh, S. M. (2009). Haptoglobin phenotype and apolipoprotein E polymorphism: Relationship to posttraumatic seizures and neuropsychological functioning after traumatic brain injury. Epilepsy & Behavior, 16, 501–506. - PMC - PubMed
-
- Aoki, K. , Uchihara, T. , Sanjo, N. , Nakamura, A. , Ikeda, K. , Tsuchiya, K. , & Wakayama, Y. (2003). Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke, 34, 875–880. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

