Borate esters: Simple catalysts for the sustainable synthesis of complex amides
- PMID: 28948222
- PMCID: PMC5609808
- DOI: 10.1126/sciadv.1701028
Borate esters: Simple catalysts for the sustainable synthesis of complex amides
Abstract
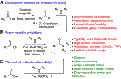
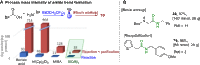
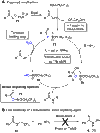
Chemical reactions for the formation of amide bonds are among the most commonly used transformations in organic chemistry, yet they are often highly inefficient. A novel protocol for amidation using a simple borate ester catalyst is reported. The process presents significant improvements over other catalytic amidation methods in terms of efficiency and safety, with an unprecedented substrate scope including functionalized heterocycles and even unprotected amino acids. The method was used to access a wide range of functionalized amide derivatives, including pharmaceutically relevant targets, important synthetic intermediates, a catalyst, and a natural product.
Figures




References
-
- Pattabiraman V. R., Bode J. W., Rethinking amide bond synthesis. Nature 480, 471–479 (2011). - PubMed
-
- Dunetz J. R., Magano J., Weisenburger G. A., Large-scale applications of amide coupling reagents for the synthesis of pharmaceuticals. Org. Process Res. Dev. 20, 140–177 (2016).
-
- Constable D. J. C., Dunn P. J., Hayler J. D., Humphrey G. R., Leazer J. L. Jr, Linderman R. J., Lorenz K., Manley J., Pearlman B. A., Wells A., Zaks A.. Zhang T. Y., Key green chemistry research areas—A perspective from pharmaceutical manufacturers. Green Chem. 9, 411–420 (2007).
-
- Chan W.-K., Ho C.-M., Wong M.-K., Che C.-M., Oxidative amide synthesis and N-terminal α-amino group ligation of peptides in aqueous medium. J. Am. Chem. Soc. 128, 14796–14797 (2006). - PubMed
-
- Gunanathan C., Ben-David Y., Milstein D., Direct synthesis of amides from alcohols and amines with liberation of H2. Science 317, 790–792 (2007). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

