Discrepancy Between Clinical and Pathologic Nodal Status of Esophageal Cancer and Impact on Prognosis and Therapeutic Strategy
- PMID: 28948524
- PMCID: PMC5670185
- DOI: 10.1245/s10434-017-6088-8
Discrepancy Between Clinical and Pathologic Nodal Status of Esophageal Cancer and Impact on Prognosis and Therapeutic Strategy
Abstract
Background: The impact of discrepancies between clinical (c) and pathologic (p) stages of esophageal cancer remains a poorly understood issue. This study aimed to compare the prognosis of patient groups treated by primary surgery including clinical N0/pathologic N0 (cN0pN0), clinical N0/pathologic N+ (cN0pN+), clinical N+/pathologic N0 (cN+pN0), and clinical N+/pathologic N+ (cN+pN+).
Methods: Data were collected from 30 European centers during the years 2000 to 2010. Among 2944 recruited patients, 1554 patients receiving primary surgery met the inclusion criteria including 613 cN0pN0, 403 cN0pN+, 220 cN+pN0, and 318 cN+pN+ patients. Analyses with adjustment of the propensity score were used to compensate for differences in baseline characteristics.
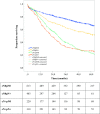
Results: Clinical T stages 3 and 4 were increased in cN+pN+ (73.0%), cN0pN+ (49.6%), and cN+pN0 (51.8%) compared with cN0pN0 (32.8%). Compared with cN0pN0, cN+pN+ and cN0pN+ showed an increase in the proportion of adenocarcinoma histologic subtype, poor tumor differentiation, pathologic T3 and T4 stages, and R1/2 resection margin. Adjusted 5-year overall survival (hazard ratio [HR] 3.12; 95% confidence interval [CI] 2.57-3.78; P < 0.001) and event-free survival (HR 2.87; 95% CI 2.39-3.45; P < 0.001) were significantly reduced in cN0pN+ compared with cN0pN0. No significant differences in 5-year overall survival or event-free survival between cN0pN+ and cN+pN+ were observed. Regression analysis identified an association of distal tumor location, advanced clinical T stage, and poor tumor differentiation with pN+ disease.
Conclusions: This large multicenter study showed that cN0pN+ has a prognosis similar to that of cN+pN+ and worse than that of cN0pN0. Patients with clinical N0 disease but risk factors for pathologic N+ disease may benefit from neoadjuvant therapy before surgery.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous