Identification of highly-protective combinations of Plasmodium vivax recombinant proteins for vaccine development
- PMID: 28949293
- PMCID: PMC5655538
- DOI: 10.7554/eLife.28673
Identification of highly-protective combinations of Plasmodium vivax recombinant proteins for vaccine development
Abstract
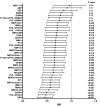
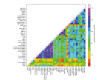
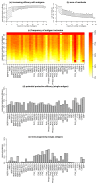
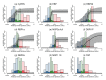
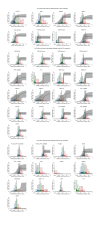
The study of antigenic targets of naturally-acquired immunity is essential to identify and prioritize antigens for further functional characterization. We measured total IgG antibodies to 38 P. vivax antigens, investigating their relationship with prospective risk of malaria in a cohort of 1-3 years old Papua New Guinean children. Using simulated annealing algorithms, the potential protective efficacy of antibodies to multiple antigen-combinations, and the antibody thresholds associated with protection were investigated for the first time. High antibody levels to multiple known and newly identified proteins were strongly associated with protection (IRR 0.44-0.74, p<0.001-0.041). Among five-antigen combinations with the strongest protective effect (>90%), EBP, DBPII, RBP1a, CyRPA, and PVX_081550 were most frequently identified; several of them requiring very low antibody levels to show a protective association. These data identify individual antigens that should be prioritized for further functional testing and establish a clear path to testing a multicomponent P. vivax vaccine.
Keywords: IgG antibody; Plasmodium vivax; clinical malaria; epidemiology; global health; natural immunity; protection; vaccine.
Conflict of interest statement
No competing interests declared.
Figures
References
-
- Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmüller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kaboré B, Sombié O, Guiguemdé RT, Ouédraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Otsyula N, Gondi S, Otieno A, Owira V, Oguk E, Odongo G, Woods JB, Ogutu B, Njuguna P, Chilengi R, Akoo P, Kerubo C, Maingi C, Lang T, Olotu A, Bejon P, Marsh K, Mwambingu G, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Dosoo D, Asante I, Adjei G, Kwara E, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Mahende C, Liheluka E, Malle L, Lemnge M, Theander TG, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Sarfo A, Agyekum A, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Tembo T, Tegha G, Tsidya M, Kilembe J, Chawinga C, Ballou WR, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Olivier A, Vekemans J, Carter T, Kaslow D, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P, RTS,S Clinical Trials Partnership A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. The New England journal of medicine. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. - DOI - PMC - PubMed
-
- Ahmed Ismail H, Tijani MK, Langer C, Reiling L, White MT, Beeson JG, Wahlgren M, Nwuba R, Persson KE. Subclass responses and their half-lives for antibodies against EBA175 and PfRh2 in naturally acquired immunity against Plasmodium falciparum malaria. Malaria Journal. 2014;13:425. doi: 10.1186/1475-2875-13-425. - DOI - PMC - PubMed
-
- Alves E, Salman AM, Leoratti F, Lopez-Camacho C, Viveros-Sandoval ME, Lall A, El-Turabi A, Bachmann MF, Hill AV, Janse CJ, Khan SM, Reyes-Sandoval A. Evaluation of plasmodium vivax cell-traversal protein for ookinetes and sporozoites as a preerythrocytic P. vivax vaccine. Clinical and Vaccine Immunology. 2017;24:e00501-16. doi: 10.1128/CVI.00501-16. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

