Calcium supplementation commencing before or early in pregnancy, or food fortification with calcium, for preventing hypertensive disorders of pregnancy
- PMID: 28949421
- PMCID: PMC6483745
- DOI: 10.1002/14651858.CD011192.pub2
Calcium supplementation commencing before or early in pregnancy, or food fortification with calcium, for preventing hypertensive disorders of pregnancy
Update in
-
Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy.Cochrane Database Syst Rev. 2019 Sep 16;9(9):CD011192. doi: 10.1002/14651858.CD011192.pub3. Cochrane Database Syst Rev. 2019. PMID: 31523806 Free PMC article.
Abstract
Background: Pre-eclampsia is considerably more prevalent in low- than high-income countries. One possible explanation for this discrepancy is dietary differences, particularly calcium deficiency. Calcium supplementation in the second half of pregnancy reduces the serious consequences of pre-eclampsia and is recommended by the World Health Organization (WHO) for women with low dietary calcium intake, but has limited effect on the overall risk of pre-eclampsia. It is important to establish whether calcium supplementation before and in early pregnancy has added benefit. Such evidence would be justification for population-level fortification of staple foods with calcium.
Objectives: To determine the effect of calcium supplementation or food fortification with calcium, commenced before or early in pregnancy and continued at least until mid-pregnancy, on pre-eclampsia and other hypertensive disorders, maternal morbidity and mortality, as well as fetal and neonatal outcomes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Trials Register (10 August 2017), PubMed (29 June 2017), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (10 August 2017) and reference lists of retrieved studies.
Selection criteria: Randomised controlled trials of calcium supplementation or food fortification which include women of child bearing age not yet pregnant, or in early pregnancy. Cluster-RCTs, quasi-RCTs and trials published in abstract form only would have been eligible for inclusion in this review but none were identified. Cross-over designs are not appropriate for this intervention.The scope of this review is to consider interventions including calcium supplementation with or without additional supplements or treatments, compared with placebo or no intervention.
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results: This review is based on one RCT (involving 60 women) which looked at calcium plus additional supplements versus control. The women (who had low antioxidant status) were in the early stages of pregnancy. We did not identify any studies where supplementation commenced pre-pregnancy. Another RCT comparing calcium versus placebo is ongoing but not yet complete. We did not identify any studies looking at any of our other planned comparisons. Calcium plus antioxidants and other supplements versus placeboWe included one small study (involving 60 women with low antioxidant levels) which was conducted in an academic hospital in Indondesia. The study was at low risk of bias for all domains with the exception of selective reporting, for which it was unclear. Women in the intervention group received calcium (800 mg) plus N-acetylcysteine (200 mg), Cu (2 mg), Zn (15 mg), Mn (0.5 mg) and selenium (100 mcg) and vitamins A (1000 IU), B6 (2.2 mg), B12 (2.2 mcg), C (200 mg), and E (400 IU) versus the placebo control group of women who received similar looking tablets containing iron and folic acid. Both groups received iron (30 mg) and folic acid (400 mcg). Tablets were taken twice daily from eight to 12 weeks of gestation and then throughout pregnancy.The included study found that calcium supplementation plus antioxidants and other supplements may slightly reduce pre-eclampsia (gestational hypertension and proteinuria) (risk ratio (RR) 0.24, 95% confidence interval (CI) 0.06 to 1.01; low-quality evidence), but this is uncertain due to wide confidence intervals just crossing the line of no effect, and small sample size. It appears that earlypregnancy loss before 20 weeks' gestation (RR 0.06, 95% CI 0.00 to 1.04; moderate-quality evidence) may be slightly reduced by calcium plus antioxidants and other supplements, but this outcome also has wide confidence intervals, which just cross the line of no effect. Very few events were reported under the composite outcome, severe maternal morbidity and mortality index and no clear difference was seen between groups (RR 0.36, 95% CI 0.04 to 3.23; low-quality evidence). However, the included study observed a reduction in the composite outcome pre-eclampsia and/or pregnancy loss at any gestational age (RR 0.13, 95% CI 0.03 to 0.50; moderate-quality evidence), and pregnancy loss/stillbirth at any gestational age (RR 0.06, 95% CI 0.00 to 0.92; moderate-quality evidence) in the calcium plus antioxidant/supplement group.Other outcomes reported (placental abruption, severe pre-eclampsia and preterm birth (less than 37 weeks' gestation)) were too infrequent for meaningful analysis. No data were reported for the outcomes caesarean section, birthweight < 2500 g, Apgar score less than seven at five minutes, death or admission to neonatal intensive care unit (ICU), or pregnancy loss, stillbirth or neonatal death before discharge from hospital.
Authors' conclusions: The results of this review are based on one small study in which the calcium intervention group also received antioxidants and other supplements. Therefore, we are uncertain whether any of the effects observed in the study were due to calcium supplementation or not. The evidence in this review was graded low to moderate due to imprecision. There is insufficient evidence on the effectiveness or otherwise of pre- or early-pregnancy calcium supplementation, or food fortification for preventing hypertensive disorders of pregnancy.Further research is needed to determine whether pre- or early-pregnancy supplementation, or food fortification with calcium is associated with a reduction in adverse pregnancy outcomes such as pre-eclampsia and pregnancy loss. Such studies should be adequately powered, limited to calcium supplementation, placebo-controlled, and include relevant outcomes such as those chosen for this review.There is one ongoing study of calcium supplementation alone versus placebo and this may provide additional evidence in future updates.
Conflict of interest statement
Justus Hofmeyr: is an investigator on a trial could potentially be included in this review in future updates ‐ however, he will not be involved in any decisions relating to the trial. Two external experienced systematic reviewers, who are not directly involved with the trial, will evaluate the trial for inclusion, assess risk of bias, and carry out data extraction.
Sarah Manyame is an investigator on a trial could potentially be included in this review in future updates ‐ however, she will not be involved in any decisions relating to the trial. Two external experienced systematic reviewers, who are not directly involved with the trial, will evaluate the trial for inclusion, assess risk of bias, and carry out data extraction.
Figures
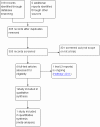









Similar articles
-
Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes.Cochrane Database Syst Rev. 2018 Jul 24;7(7):CD010564. doi: 10.1002/14651858.CD010564.pub2. Cochrane Database Syst Rev. 2018. PMID: 30039871 Free PMC article.
-
Vitamin supplementation for preventing miscarriage.Cochrane Database Syst Rev. 2016 May 6;2016(5):CD004073. doi: 10.1002/14651858.CD004073.pub4. Cochrane Database Syst Rev. 2016. PMID: 27150280 Free PMC article.
-
Vitamin D supplementation for women during pregnancy.Cochrane Database Syst Rev. 2024 Jul 30;7(7):CD008873. doi: 10.1002/14651858.CD008873.pub5. Cochrane Database Syst Rev. 2024. PMID: 39077939 Free PMC article.
-
Vitamin D supplementation for women during pregnancy.Cochrane Database Syst Rev. 2016 Jan 14;(1):CD008873. doi: 10.1002/14651858.CD008873.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2019 Jul 26;7:CD008873. doi: 10.1002/14651858.CD008873.pub4. PMID: 26765344 Updated.
-
Antenatal corticosteroids prior to planned caesarean at term for improving neonatal outcomes.Cochrane Database Syst Rev. 2021 Dec 22;12(12):CD006614. doi: 10.1002/14651858.CD006614.pub4. Cochrane Database Syst Rev. 2021. PMID: 34935127 Free PMC article.
Cited by
-
Dietary guideline adherence during preconception and pregnancy: A systematic review.Matern Child Nutr. 2020 Apr;16(2):e12916. doi: 10.1111/mcn.12916. Epub 2019 Dec 2. Matern Child Nutr. 2020. PMID: 31793249 Free PMC article.
-
Impact of water fortification with calcium on calcium intake in different countries: a simulation study.Public Health Nutr. 2022 Feb;25(2):344-357. doi: 10.1017/S1368980020002232. Epub 2020 Aug 3. Public Health Nutr. 2022. PMID: 32744224 Free PMC article.
-
Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews.Cochrane Database Syst Rev. 2018 Nov 14;11(11):CD012505. doi: 10.1002/14651858.CD012505.pub2. Cochrane Database Syst Rev. 2018. PMID: 30480756 Free PMC article.
-
The management of hypertension in women planning for pregnancy.Br Med Bull. 2018 Dec 1;128(1):75-84. doi: 10.1093/bmb/ldy035. Br Med Bull. 2018. PMID: 30371746 Free PMC article. Review.
-
Prepregnancy and early pregnancy calcium supplementation among women at high risk of pre-eclampsia: a multicentre, double-blind, randomised, placebo-controlled trial.Lancet. 2019 Jan 26;393(10169):330-339. doi: 10.1016/S0140-6736(18)31818-X. Lancet. 2019. PMID: 30696573 Free PMC article. Clinical Trial.
References
References to studies included in this review
-
- Rumiris D, Purwosunu Y, Wibowo N, Farina A, Sekizawa A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertension in Pregnancy 2006;25(3):241‐53. - PubMed
References to ongoing studies
-
- Hofmeyr GJ. Protocol 11PRT/4028: Long term calcium supplementation in women at high risk of pre‐eclampsia: a randomised, placebo‐controlled trial (PACTR201105000267371). Lancet.com (http://www.thelancet.com/protocol‐reviews/11PRT‐4028)2011.
- Hofmeyr GJ, Seuc AH, Betrán AP, Purnat TD, Ciganda A, Munjanja SP, et al. The effect of calcium supplementation on blood pressure in non‐pregnant women with previous pre‐eclampsia: an exploratory, randomized placebo controlled study. Pregnancy Hypertension 2015;5(4):273‐9. - PubMed
- PACTR201105000267371. WHO randomized trial of calcium supplementation before pregnancy to reduce recurrent pre‐eclampsia. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR2011050002... (first received 6 December 2010).
Additional references
-
- Belizan JM, Villar J. The relationship between calcium intake and edema‐, proteinuria‐, and hypertension‐getosis: an hypothesis. American Journal of Clinical Nutrition 1980;33:2202‐10. - PubMed
-
- Buppasiri P, Lumbiganon P, Thinkhamrop J, Ngamjarus C, Laopaiboon M, Medley N. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD007079.pub3] - DOI - PMC - PubMed
-
- Centeno V, Barboza GD, Marchionatti A, Rodriguez V, Talamoni NT. Molecular mechanisms triggered by low‐calcium diets. Nutritional Research Reviews 2009;22:163‐74. - PubMed
-
- Griffith LE, Guyatt GH, Cook RJ, Bucher HC, Cook DJ. The influence of dietary and nondietary calcium supplementation blood pressure: an updated metaanalysis of randomized controlled trials. American Journal of Hypertension 1999;12(1):84‐92. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

