Estrogen, through estrogen receptor 1, regulates histone modifications and chromatin remodeling during spermatogenesis in adult rats
- PMID: 28949791
- PMCID: PMC5788427
- DOI: 10.1080/15592294.2017.1382786
Estrogen, through estrogen receptor 1, regulates histone modifications and chromatin remodeling during spermatogenesis in adult rats
Abstract
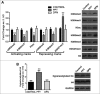
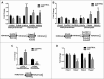
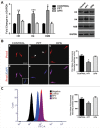
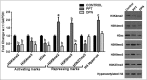
Estrogen receptors (ESR1 and ESR2) play crucial roles in various processes during spermatogenesis. To elucidate individual roles of ESRs in male fertility, we developed in vivo selective ESR agonist administration models. Adult male rats treated with ESR1 and ESR2 agonist for 60 days show spermatogenic defects leading to reduced sperm counts and fertility. While studying epigenetic changes in the male germ line that could have affected fertility, we earlier observed a decrease in DNA methylation and its machinery upon ESR2 agonist treatment. Here, we explored the effects on histone modifications, which could contribute to decreased male fertility upon ESR agonist administration. ESR1 agonist treatment affected testicular levels of histone modifications associated with active and repressed chromatin states, along with heterochromatin marks. This was concomitant with deregulation of corresponding histone modifying enzymes in the testis. In addition, there was increased retention of histones along with protamine deficiency in the caudal spermatozoa after ESR1 agonist treatment. This could be due to the observed decrease in several chromatin remodeling proteins implicated in mediating histone-to-protamine exchange during spermiogenesis. The activating and repressing histone marks in spermatozoa, which play a critical role in early embryo development, were deregulated after both the ESR agonist treatments. Together, these epigenetic defects in the male germ line could affect the spermatozoa quality and lead to the observed decrease in fertility. Our results thus highlight the importance of ESRs in regulating different epigenetic processes during spermatogenesis, which are crucial for male fertility.
Keywords: chromatin remodeling; estrogen receptor; histone modifications; spermatogenesis; spermatozoa.
Figures


Similar articles
-
Differential roles of estrogen receptors, ESR1 and ESR2, in adult rat spermatogenesis.Mol Cell Endocrinol. 2016 Jun 15;428:89-100. doi: 10.1016/j.mce.2016.03.024. Epub 2016 Mar 19. Mol Cell Endocrinol. 2016. PMID: 27004961
-
Estrogen signaling, through estrogen receptor β, regulates DNA methylation and its machinery in male germ line in adult rats.Epigenetics. 2017 Jun 3;12(6):476-483. doi: 10.1080/15592294.2017.1309489. Epub 2017 Mar 31. Epigenetics. 2017. PMID: 28362134 Free PMC article.
-
The effects of chemotherapy with bleomycin, etoposide, and cis-platinum (BEP) on rat sperm chromatin remodeling, fecundity and testicular gene expression in the progeny.Biol Reprod. 2013 Oct 10;89(4):85. doi: 10.1095/biolreprod.113.110759. Print 2013 Oct. Biol Reprod. 2013. PMID: 23986570
-
The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis.Asian J Androl. 2015 Nov-Dec;17(6):948-53. doi: 10.4103/1008-682X.150844. Asian J Androl. 2015. PMID: 25814158 Free PMC article. Review.
-
Epigenetics, spermatogenesis and male infertility.Mutat Res. 2011 May-Jun;727(3):62-71. doi: 10.1016/j.mrrev.2011.04.002. Epub 2011 Apr 16. Mutat Res. 2011. PMID: 21540125 Review.
Cited by
-
Sex-Bias in Irritable Bowel Syndrome: Linking Steroids to the Gut-Brain Axis.Front Endocrinol (Lausanne). 2021 May 19;12:684096. doi: 10.3389/fendo.2021.684096. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34093447 Free PMC article. Review.
-
Testosterone Retention Mechanism in Sertoli Cells: A Biochemical Perspective.Open Biochem J. 2018 Jun 29;12:103-112. doi: 10.2174/1874091X01812010103. eCollection 2018. Open Biochem J. 2018. PMID: 30069251 Free PMC article. Review.
-
Clinician's guide to the management of azoospermia induced by exogenous testosterone or anabolic-androgenic steroids.Asian J Androl. 2025 May 1;27(3):330-341. doi: 10.4103/aja2024104. Epub 2025 Jan 17. Asian J Androl. 2025. PMID: 39820213 Free PMC article. Review.
-
Identification of the TSSK4 Alternative Spliceosomes and Analysis of the Function of the TSSK4 Protein in Yak (Bos grunniens).Animals (Basel). 2022 May 27;12(11):1380. doi: 10.3390/ani12111380. Animals (Basel). 2022. PMID: 35681843 Free PMC article.
-
Missing Information from the Estrogen Receptor Puzzle: Where Are They Localized in Bull Reproductive Tissues and Spermatozoa?Cells. 2020 Jan 10;9(1):183. doi: 10.3390/cells9010183. Cells. 2020. PMID: 31936899 Free PMC article.
References
-
- Peters AH, O'Carroll D, Scherthan H, Mechtler K, Sauer S, Schöfer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, et al.. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323-37. doi:10.1016/S0092-8674(01)00542-6. PMID:11701123 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
