Impact of Salmonid alphavirus infection in diploid and triploid Atlantic salmon (Salmo salar L.) fry
- PMID: 28949966
- PMCID: PMC5614425
- DOI: 10.1371/journal.pone.0179192
Impact of Salmonid alphavirus infection in diploid and triploid Atlantic salmon (Salmo salar L.) fry
Abstract
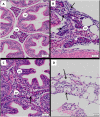
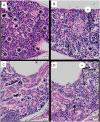
With increasing interest in the use of triploid salmon in commercial aquaculture, gaining an understanding of how economically important pathogens affect triploid stocks is important. To compare the susceptibility of diploid and triploid Atlantic salmon (Salmo salar L.) to viral pathogens, fry were experimentally infected with Salmonid alphavirus sub-type 1 (SAV1), the aetiological agent of pancreas disease (PD) affecting Atlantic salmon aquaculture in Europe. Three groups of fry were exposed to the virus via different routes of infection: intraperitoneal injection (IP), bath immersion, or cohabitation (co-hab) and untreated fry were used as a control group. Mortalities commenced in the co-hab challenged diploid and triploid fish from 11 days post infection (dpi), and the experiment was terminated at 17 dpi. Both diploid and triploid IP challenged groups had similar levels of cumulative mortality at the end of the experimental period (41.1% and 38.9% respectively), and these were significantly higher (p < 0.01) than for the other challenge routes. A TaqMan-based quantitative PCR was used to assess SAV load in the heart, a main target organ of the virus, and also liver, which does not normally display any pathological changes during clinical infections, but exhibited severe degenerative lesions in the present study. The median viral RNA copy number was higher in diploid fish compared to triploid fish in both the heart and the liver of all three challenged groups. However, a significant statistical difference (p < 0.05) was only apparent in the liver of the co-hab groups. Diploid fry also displayed significantly higher levels of pancreatic and myocardial degeneration than triploids. This study showed that both diploid and triploid fry are susceptible to experimental SAV1 infection. The lower virus load seen in the triploids compared to the diploids may possibly be related to differences in cell metabolism between the two groups, however, further investigation is necessary to confirm this and also to assess the outcome of PD outbreaks in other developmental stages of the fish when maintained in commercial production systems.
Conflict of interest statement
Figures





Similar articles
-
Triploid atlantic salmon (Salmo salar L.) post-smolts accumulate prevalence more slowly than diploid salmon following bath challenge with salmonid alphavirus subtype 3.PLoS One. 2017 Apr 12;12(4):e0175468. doi: 10.1371/journal.pone.0175468. eCollection 2017. PLoS One. 2017. PMID: 28403165 Free PMC article.
-
Effects of ploidy and salmonid alphavirus infection on the skin and gill microbiome of Atlantic salmon (Salmo salar).PLoS One. 2021 Feb 19;16(2):e0243684. doi: 10.1371/journal.pone.0243684. eCollection 2021. PLoS One. 2021. PMID: 33606747 Free PMC article.
-
Atlantic salmon (Salmo salar L.) post-smolts challenged two or nine weeks after seawater-transfer show differences in their susceptibility to salmonid alphavirus subtype 3 (SAV3).Virol J. 2016 Apr 11;13:66. doi: 10.1186/s12985-016-0520-8. Virol J. 2016. PMID: 27068518 Free PMC article.
-
The epidemiology of pancreas disease in salmonid aquaculture: a summary of the current state of knowledge.J Fish Dis. 2017 Jan;40(1):141-155. doi: 10.1111/jfd.12478. Epub 2016 May 2. J Fish Dis. 2017. PMID: 27136332 Review.
-
Alphavirus infections in salmonids--a review.J Fish Dis. 2007 Sep;30(9):511-31. doi: 10.1111/j.1365-2761.2007.00848.x. J Fish Dis. 2007. PMID: 17718707 Review.
Cited by
-
Non-Lethal Sequential Individual Monitoring of Viremia in Relation to DNA Vaccination in Fish-Example Using a Salmon Alphavirus DNA Vaccine in Atlantic Salmon Salmo salar.Vaccines (Basel). 2021 Feb 17;9(2):163. doi: 10.3390/vaccines9020163. Vaccines (Basel). 2021. PMID: 33671162 Free PMC article.
-
Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU)2016/429): Infection with salmonid alphavirus (SAV).EFSA J. 2023 Oct 30;21(10):e08327. doi: 10.2903/j.efsa.2023.8327. eCollection 2023 Oct. EFSA J. 2023. PMID: 37908450 Free PMC article.
-
Entry receptors - the gateway to alphavirus infection.J Clin Invest. 2023 Jan 17;133(2):e165307. doi: 10.1172/JCI165307. J Clin Invest. 2023. PMID: 36647825 Free PMC article. Review.
-
Nanopore sequencing provides snapshots of the genetic variation within salmonid alphavirus-3 (SAV3) during an ongoing infection in Atlantic salmon (Salmo salar) and brown trout (Salmo trutta).Vet Res. 2024 Sep 3;55(1):106. doi: 10.1186/s13567-024-01349-z. Vet Res. 2024. PMID: 39227887 Free PMC article.
-
Comparative ploidy response to experimental hydrogen peroxide exposure in Atlantic salmon (Salmo salar).Fish Shellfish Immunol. 2018 Oct;81:354-367. doi: 10.1016/j.fsi.2018.07.017. Epub 2018 Jul 25. Fish Shellfish Immunol. 2018. PMID: 30012493 Free PMC article.
References
-
- Benfey TJ. Effectiveness of triploidy as a management tool for reproductive containment of farmed fish: Atlantic salmon (Salmo salar) as a case study. Rev Aquac. 2015; n/a–n/a. 10.1111/raq.12092 - DOI
-
- Nichols KM. Clonal Lines and Chromosome Manipulation for Aquaculture Research and Production. Molecular Research in Aquaculture. 2009. pp. 195–216. 10.1002/9780813807379.ch8 - DOI
-
- Leclercq E, Taylor JF, Fison D, Fjelldal PG, Diez-Padrisa M, Hansen T, et al. Comparative seawater performance and deformity prevalence in out-of-season diploid and triploid Atlantic salmon (Salmo salar) post-smolts. Comp Biochem Physiol A Mol Integr Physiol. 2011;158: 116–25. 10.1016/j.cbpa.2010.09.018 - DOI - PubMed
-
- Taylor JF, Sambraus F, Mota-Velasco J, Guy DR, Hamilton A, Hunter D, et al. Ploidy and family effects on Atlantic salmon (Salmo salar) growth, deformity and harvest quality during a full commercial production cycle. Aquaculture. 2013;410–411: 41–50. 10.1016/j.aquaculture.2013.06.004 - DOI
-
- Sadler J, Wells RMG, Pankhurst PM, Pankhurst NW. Blood oxygen transport, rheology and haematological responses to confinement stress in diploid and triploid Atlantic salmon, Salmo salar. Aquaculture. 2000;184: 349–361. 10.1016/S0044-8486(99)00321-X - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

