Validation of participant eligibility for pre-exposure prophylaxis: Baseline data from the PRELUDE demonstration project
- PMID: 28950022
- PMCID: PMC5614574
- DOI: 10.1371/journal.pone.0185398
Validation of participant eligibility for pre-exposure prophylaxis: Baseline data from the PRELUDE demonstration project
Abstract
Background: In Australia, pre-exposure prophylaxis (PrEP) is targeted to individuals at high risk for HIV infection. We describe the HIV risk profile and characteristics of PRELUDE participants, and evaluate the population validity of the sample in representing high-risk gay and bisexual men (GBM) eligible for PrEP.
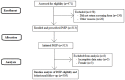
Methods: PRELUDE is an on-going, open-label, single-arm observational study. Participants were identified in clinics and screened for eligibility using a paper-based risk assessment tool which followed the New South Wales (NSW) PrEP guidelines. Selection was validated using an independent online behavioural survey, completed by study participants upon enrolment. Demographic information was analysed using descriptive statistics, and kappa tests were used to determine agreement between reporting of high-risk practices in the risk assessment and behavioural survey.
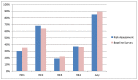
Results: During 2014-15, 471 individuals were targeted for enrolment; 341 were assessed for PrEP eligibility and 313 were enrolled. Of these, 303 (97%) identified as GBM. Overall, 85% of GBM met at least one high-risk criterion; 68% reported receptive intercourse with an HIV-positive or unknown status casual male partner, and 37% reported methamphetamine use in the three months preceding enrolment. The remaining 15% were enrolled based on medium-risk behaviours, or at the clinicians' discretion. We found an 82% total agreement between self-reported high-risk behaviour and clinicians' categorisation of GBM as being at high risk for HIV based on PrEP eligibility criteria.
Conclusions: Behavioural eligibility criteria used by clinicians successfully identified individuals at high risk for HIV infection. This targeted approach ensures that the greatest public health and HIV prevention benefits can be derived in a setting without universal access to PrEP.
Conflict of interest statement
Figures

References
-
- Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O'Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12): 1973–83. doi: 10.1097/QAD.0000000000001145 - DOI - PMC - PubMed
-
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27): 2587–99. doi: 10.1056/NEJMoa1011205 - DOI - PMC - PubMed
-
- Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23): 2237–46. doi: 10.1056/NEJMoa1506273 - DOI - PubMed
-
- McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2015;387(10013): 53–60. doi: 10.1016/S0140-6736(15)00056-2 - DOI - PMC - PubMed
-
- Baeten JM, Heffron R, Kidoguchi L, Mugo N, Katabira E, Bukusi E, et al. Integrated delivery of PrEP and ART results in sustained near elimination of HIV transmission in African HIV serodiscordant couples: final results from The Partners Demonstration Project. AIDS2016; 2016. Durban (South Africa).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

