Assessment of MALDI-TOF MS biotyping for Borrelia burgdorferi sl detection in Ixodes ricinus
- PMID: 28950023
- PMCID: PMC5614582
- DOI: 10.1371/journal.pone.0185430
Assessment of MALDI-TOF MS biotyping for Borrelia burgdorferi sl detection in Ixodes ricinus
Abstract
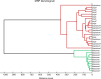
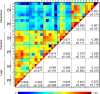
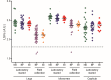
Matrix Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) has been demonstrated to be useful for tick identification at the species level. More recently, this tool has been successfully applied for the detection of bacterial pathogens directly in tick vectors. The present work has assessed the detection of Borrelia burgdorferi sensu lato in Ixodes ricinus tick vector by MALDI-TOF MS. To this aim, experimental infection model of I. ricinus ticks by B. afzelii was carried out and specimens collected in the field were also included in the study. Borrelia infectious status of I. ricinus ticks was molecularly controlled using half-idiosome to classify specimens. Among the 39 ticks engorged on infected mice, 14 were confirmed to be infected by B. afzelii. For field collection, 14.8% (n = 12/81) I. ricinus ticks were validated molecularly as infected by B. burgdorferi sl. To determine the body part allowing the detection of MS protein profile changes between non-infected and B. afzelii infected specimens, ticks were dissected in three compartments (i.e. 4 legs, capitulum and half-idiosome) prior to MS analysis. Highly reproducible MS spectra were obtained for I. ricinus ticks according to the compartment tested and their infectious status. However, no MS profile change was found when paired body part comparison between non-infected and B. afzelii infected specimens was made. Statistical analyses did not succeed to discover, per body part, specific MS peaks distinguishing Borrelia-infected from non-infected ticks whatever their origins, laboratory reared or field collected. Despite the unsuccessful of MALDI-TOF MS to classify tick specimens according to their B. afzelii infectious status, this proteomic tool remains a promising method for rapid, economic and accurate identification of tick species. Moreover, the singularity of MS spectra between legs and half-idiosome of I. ricinus could be used to reinforce this proteomic identification by submission of both these compartments to MS.
Conflict of interest statement
Figures



References
-
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet Lond Engl. 2012;379: 461–473. - PubMed
-
- Cutler SJ, Ruzic-Sabljic E, Potkonjak A. Emerging borreliae—Expanding beyond Lyme borreliosis. Mol Cell Probes. 2016; 22–27. doi: 10.1016/j.mcp.2016.08.003 - DOI - PubMed
-
- Ferquel E, Garnier M, Marie J, Bernede-Bauduin C, Baranton G, Perez-Eid C, et al. Prevalence of Borrelia burgdorferi sensu lato and Anaplasmataceae members in Ixodes ricinus ticks in Alsace, a focus of Lyme Borreliosis endemicity in France. Appl Environ Microbiol. 2006;72: 3074–3078. doi: 10.1128/AEM.72.4.3074-3078.2006 - DOI - PMC - PubMed
-
- Yssouf A, Almeras L, Raoult D, Parola P. Emerging tools for identification of arthropod vectors. Future Microbiol. 2016;11: 549–566. doi: 10.2217/fmb.16.5 - DOI - PubMed
-
- Karger A, Kampen H, Bettin B, Dautel H, Ziller M, Hoffmann B, et al. Species determination and characterization of developmental stages of ticks by whole-animal matrix-assisted laser desorption/ionization mass spectrometry. Ticks Tick-Borne Dis. 2012;3: 78–89. doi: 10.1016/j.ttbdis.2011.11.002 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

