ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe
- PMID: 28950323
- PMCID: PMC5834140
- DOI: 10.1093/annonc/mdx521
ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe
Abstract
Background: The availability and affordability of safe, effective, high-quality, affordable anticancer therapies are a core requirement for effective national cancer control plans.
Method: Online survey based on a previously validated approach. The aims of the study were to evaluate (i) the availability on national formulary of licensed antineoplastic medicines across the globe, (ii) patient out-of-pocket costs for the medications, (iii) the actual availability of the medication for a patient with a valid prescription, (iv) information relating to possible factors adversely impacting the availability of antineoplastic agents and (v) the impact of the country's level of economic development on these parameters. A total of 304 field reporters from 97 countries were invited to participate. The preliminary set of data was posted on the ESMO website for open peer review and amendments have been incorporated into the final report.
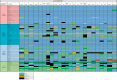
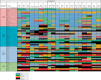
Results: Surveys were submitted by 135 reporters from 63 countries and additional peer-review data were submitted by 54 reporters from 19 countries. There are substantial differences in the formulary availability, out-of-pocket costs and actual availability for many anticancer medicines. The most substantial issues are in lower-middle- and low-income countries. Even among medications on the WHO Model List of Essential Medicines (EML) the discrepancies are profound and these relate to high out-of-pocket costs (in low-middle-income countries 32.0% of EML medicines are available only at full cost and 5.2% are not available at all, and for low-income countries, the corresponding figures are even worse at 57.7% and 8.3%, respectively).
Conclusions: There is wide global variation in formulary availability, out-of-pocket expenditures and actual availability for most licensed anticancer medicines. Low- and low-middle-income countries have significant lack of availability and high out-of-pocket expenditures for cancer medicines on the WHO EML, with much less availability of new, more expensive targeted agents compared with high-income countries.
Keywords: ESMO; WHO Model List of Essential Medicines; anticancer medicines; antineoplastic medicines; public health; public policy.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures




Comment in
-
Policy and governance solutions for ensuring equitable access to cancer medicines in low- and middle-income countries.Ann Transl Med. 2018 Jun;6(11):224. doi: 10.21037/atm.2018.04.26. Ann Transl Med. 2018. PMID: 30023387 Free PMC article. No abstract available.
Similar articles
-
ESMO European Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in Europe.Ann Oncol. 2016 Aug;27(8):1423-43. doi: 10.1093/annonc/mdw213. Ann Oncol. 2016. PMID: 27457309
-
ESMO Global Consortium Study on the availability, out-of-pocket costs, and accessibility of cancer medicines: 2023 update.Ann Oncol. 2025 Mar;36(3):247-262. doi: 10.1016/j.annonc.2024.12.005. Epub 2025 Jan 16. Ann Oncol. 2025. PMID: 39818519
-
Access to cancer medicines deemed essential by oncologists in 82 countries: an international, cross-sectional survey.Lancet Oncol. 2021 Oct;22(10):1367-1377. doi: 10.1016/S1470-2045(21)00463-0. Epub 2021 Sep 21. Lancet Oncol. 2021. PMID: 34560006 Free PMC article.
-
Availability and accessibility of cytotoxic medicines in the WHO model list of essential medicines for childhood cancer in low and lower-middle- income countries: a systematic review.BMC Cancer. 2025 Jan 31;25(1):181. doi: 10.1186/s12885-025-13586-2. BMC Cancer. 2025. PMID: 39891074 Free PMC article.
-
Cancer medicines in Asia and Asia-Pacific: What is available, and is it effective enough?ESMO Open. 2019 Jul 17;4(4):e000483. doi: 10.1136/esmoopen-2018-000483. eCollection 2019. ESMO Open. 2019. PMID: 31423334 Free PMC article. Review.
Cited by
-
The current situation regarding the availability and accessibility of anticancer drugs for breast cancer in the Peruvian public health systems.Ecancermedicalscience. 2021 Apr 27;15:1224. doi: 10.3332/ecancer.2021.1224. eCollection 2021. Ecancermedicalscience. 2021. PMID: 34158828 Free PMC article.
-
Prices and Affordability of Essential Medicines in 72 Low-, Middle-, and High-Income Markets.JAMA Health Forum. 2025 Aug 1;6(8):e252043. doi: 10.1001/jamahealthforum.2025.2043. JAMA Health Forum. 2025. PMID: 40815523 Free PMC article.
-
Evaluating essential medicines for treating childhood cancers: availability, price and affordability study in Ghana.BMC Cancer. 2021 Jun 10;21(1):683. doi: 10.1186/s12885-021-08435-x. BMC Cancer. 2021. PMID: 34112117 Free PMC article.
-
Perception About Factors Affecting Patient Adherence With Cardiac Medicines: A Cross-Sectional Study.Health Sci Rep. 2025 Mar 5;8(3):e70532. doi: 10.1002/hsr2.70532. eCollection 2025 Mar. Health Sci Rep. 2025. PMID: 40051493 Free PMC article.
-
Global Cancer Drug Development-A Report From the 2022 Accelerating Anticancer Agent Development and Validation Meeting.JCO Glob Oncol. 2023 Sep;9:e2300294. doi: 10.1200/GO.23.00294. JCO Glob Oncol. 2023. PMID: 37944089 Free PMC article.
References
-
- United Nations General Assembly. Political declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases. New York: United Nations; 2011; http://www.who.int/nmh/events/un_ncd_summit2011/political_declaration_en...: pp13 (August 2017, date last accessed).
-
- World Health Assembly S-S. Follow-up to the political declaration of the high-level meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases. WHA6610, 2013. http://apps.who.int/gb/ebwha/pdf_files/WHA66/A66_R10-en.pdf?ua=12013; pp55 (August 2017, date last accessed).
-
- Seventieth World Health Assembly. Cancer prevention and control in the context of an integrated approach (A70/32). Geneva: World Health Organization; 2017; pp6 http://apps.who.int/gb/ebwha/pdf_files/WHA70/A70_32-en.pdf (August 2017, date last accessed).
-
- World Health Organization. The selection and use of essential medicines. World Health Organization Technical Report Series 2007; 1–162 (back cover). - PubMed
-
- World Health Organization. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2015 in WHO Technical Report Series. Geneva: World Health Association 2015; 553.http://apps.who.int/iris/bitstream/10665/189763/189761/9789241209946_eng... (August 2017, date last accessed).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

