Dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis of safety data from phase IIb/III clinical trials
- PMID: 28950419
- PMCID: PMC5836959
- DOI: 10.1111/dom.13124
Dapagliflozin in patients with type 2 diabetes mellitus: A pooled analysis of safety data from phase IIb/III clinical trials
Abstract
Aim: To evaluate the safety and tolerability of dapagliflozin, a highly selective sodium-glucose co-transporter-2 inhibitor, in patients with type 2 diabetes mellitus (T2DM).
Methods: Data were pooled from 13 placebo-controlled trials of up to 24 weeks' duration (dapagliflozin, n = 2360; placebo, n = 2295). Larger placebo-/comparator-controlled pools of 21 (≤208 weeks; dapagliflozin, n = 5936; control, n = 3403) and 30 trials (≥12 weeks; dapagliflozin, n = 9195; control, n = 4629) assessed the rare adverse events (AEs) of diabetic ketoacidosis (DKA) and lower limb amputation, respectively.
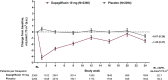
Results: Over 24 weeks, the overall incidence of AEs and serious AEs (SAEs) was similar for dapagliflozin and placebo: 60.0% vs 55.7% and 5.1% vs 5.4%, respectively. Rates of hypoglycaemia, volume depletion AEs, urinary tract infections (UTIs) and fractures were balanced between the groups. Genital infections were more frequent with dapagliflozin (5.5%) vs placebo (0.6%) and renal function AEs occurred in 3.2% vs 1.8% of patients (the most common renal AE was decreased creatinine clearance: 1.1% vs 0.7%). In the 21-study pool, 1 SAE of DKA and 3 AEs of ketonuria/metabolic acidosis occurred with dapagliflozin vs none with control; estimated combined incidence for these events was 0.03% (95% confidence interval 0.010-0.089). In the 30-study pool, lower limb amputation occurred in 8 (0.1%) and 7 (0.2%) patients receiving dapagliflozin and control, respectively.
Conclusion: The overall incidence rates of AEs and SAEs were similar in the dapagliflozin and placebo/control groups, including the incidence of hypoglycaemia, volume depletion, fractures, UTIs, amputations and DKA. Genital infections were more frequent with dapagliflozin than placebo.
Keywords: SGLT2 inhibitor; antidiabetic drug; dapagliflozin; type 2 diabetes.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
S. J. is a consultant for AstraZeneca, Janssen and Eli Lilly. J. S. has attended advisory boards and/or speaker's bureaus for Takeda, Bayer, Novartis, Merck Sharp & Dohme, Amgen, AstraZeneca, Bristol‐Myers Squibb, Novo Nordisk, Sanofi, Berlin‐Chemie, Eli Lilly, Boehringer Ingelheim, Merck, Roche, Ipsen, Pfizer, Janssen and LifeScan, and has received project‐specific research support from AstraZeneca, Takeda, Novartis, Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Novo Nordisk, Sanofi, Ipsen, Pfizer, Janssen, Servier, Eli Lilly, Apitope, Intarcia and Roche. A.S. has attended advisory boards and/or speaker's bureaus for AstraZeneca, Novo Nordisk, Eli Lilly, Sanofi, Merck Sharp & Dohme, Boehringer Ingelheim and Janssen, and has received research support from Novo Nordisk. C. J. B. received grants and personal fees from AstraZeneca, Bristol‐Myers Squibb, Sanofi and personal fees from Boehringer Ingelheim, Eli Lilly, Johnson & Johnson, Merck Sharp & Dohme, Novo Nordisk, Elcelyx and Poxel, outside of the submitted work. C. K. and A. M. L. are employees and stockholders of AstraZeneca.
Figures
Similar articles
-
Long-Term Safety of Dapagliflozin in Older Patients with Type 2 Diabetes Mellitus: A Pooled Analysis of Phase IIb/III Studies.Drugs Aging. 2016 Jul;33(7):511-22. doi: 10.1007/s40266-016-0382-1. Drugs Aging. 2016. PMID: 27357173 Free PMC article.
-
Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years.Diabetes Obes Metab. 2014 Feb;16(2):124-36. doi: 10.1111/dom.12187. Epub 2013 Aug 29. Diabetes Obes Metab. 2014. PMID: 23911013 Clinical Trial.
-
Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events.Drug Saf. 2014 Oct;37(10):815-29. doi: 10.1007/s40264-014-0213-4. Drug Saf. 2014. PMID: 25096959
-
Safety of Sodium-Glucose Co-Transporter 2 Inhibitors.Am J Cardiol. 2019 Dec 15;124 Suppl 1:S45-S52. doi: 10.1016/j.amjcard.2019.10.029. Am J Cardiol. 2019. PMID: 31741440 Review.
-
[Dapagliflozin, the first SGLT-2 inhibitor in the treatment of type 2 diabetes].Med Clin (Barc). 2013 Sep;141 Suppl 2:36-43. doi: 10.1016/S0025-7753(13)70062-9. Med Clin (Barc). 2013. PMID: 24444523 Review. Spanish.
Cited by
-
SGLT2 inhibitor treatment is not associated with an increased risk of osteoporotic fractures when compared to GLP-1 receptor agonists: A nationwide cohort study.Front Endocrinol (Lausanne). 2022 Aug 19;13:861422. doi: 10.3389/fendo.2022.861422. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060970 Free PMC article.
-
Post-transplant diabetes mellitus in patients with solid organ transplants.Nat Rev Endocrinol. 2019 Mar;15(3):172-188. doi: 10.1038/s41574-018-0137-7. Nat Rev Endocrinol. 2019. PMID: 30622369 Review.
-
Novel Approaches to Restore Pancreatic Beta-Cell Mass and Function.Handb Exp Pharmacol. 2022;274:439-465. doi: 10.1007/164_2021_474. Handb Exp Pharmacol. 2022. PMID: 34114119 Review.
-
Cost Offset of Dapagliflozin in the US Medicare Population with Cardio-Kidney Metabolic Syndrome.Adv Ther. 2024 Aug;41(8):3247-3263. doi: 10.1007/s12325-024-02919-5. Epub 2024 Jul 3. Adv Ther. 2024. PMID: 38958842 Free PMC article.
-
Diagnosis and Non-Invasive Treatment of Obesity in Adults with Type 2 Diabetes Mellitus: A Review of Guidelines.J Clin Med. 2023 Jun 30;12(13):4431. doi: 10.3390/jcm12134431. J Clin Med. 2023. PMID: 37445466 Free PMC article. Review.
References
-
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. - PubMed
-
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. - PubMed
-
- Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513–519. - PubMed
-
- Scheen AJ. Pharmacodynamics, efficacy and safety of sodium‐glucose co‐transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical