Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway
- PMID: 28950908
- PMCID: PMC5615458
- DOI: 10.1186/s12974-017-0967-6
Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway
Abstract
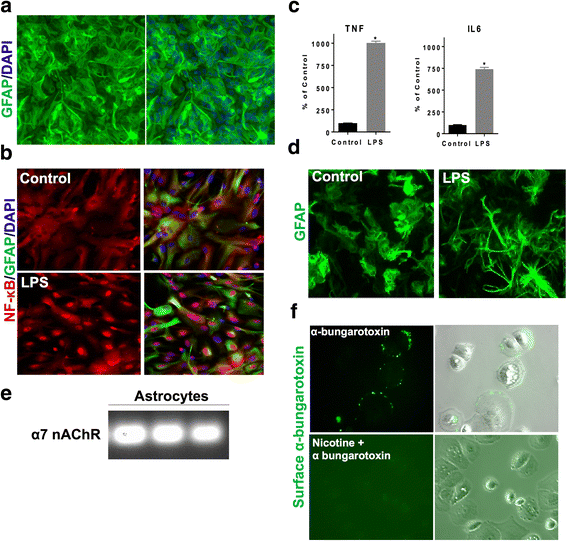
Background: α7 nicotinic acetylcholine receptors (nAChRs) are widely distributed throughout the central nervous system and are reported to have neuroprotective properties. α7 nAChRs are expressed on astrocytes, which are key regulators of neuroinflammation and oxidative stress in several neurodegenerative diseases. However, the anti-inflammatory and antioxidant properties of astroglial α7 nAChRs are not well studied. Therefore, we evaluated the role of astroglial α7 nAChR activation in neuroinflammation.
Methods: Anti-inflammatory and antioxidant effects of α7 nAChR activation were evaluated in an in vitro mouse model of neuroinflammation using lipopolysaccharide (LPS) in primary astrocyte cultures. α7 nAChR anti-inflammatory effects on the NF-κB pathway were evaluated using ELISA, gene expression analysis, immunofluorescence, and western blotting. Antioxidant effect of α7 nAChR activation on expression profiles of canonical Nrf2 target genes was examined by quantitative PCR and western blotting. The role of the Nrf2 pathway in α7 nAChR-mediated anti-inflammatory response was evaluated using Nrf2 knockout astrocytes. Brain ex vivo NF-κB luciferase signals were evaluated after treatment with an α7 nAChR agonist in lipopolysaccharide (LPS)-injected NF-κB luciferase reporter mouse model.
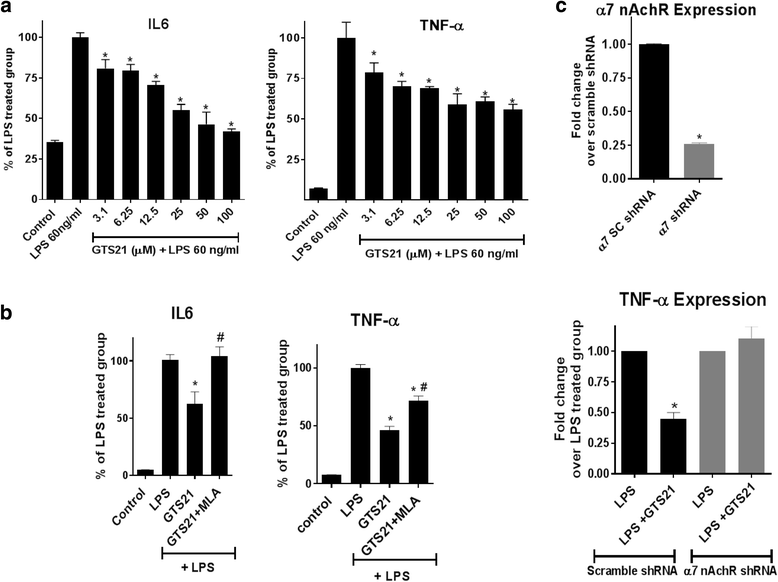
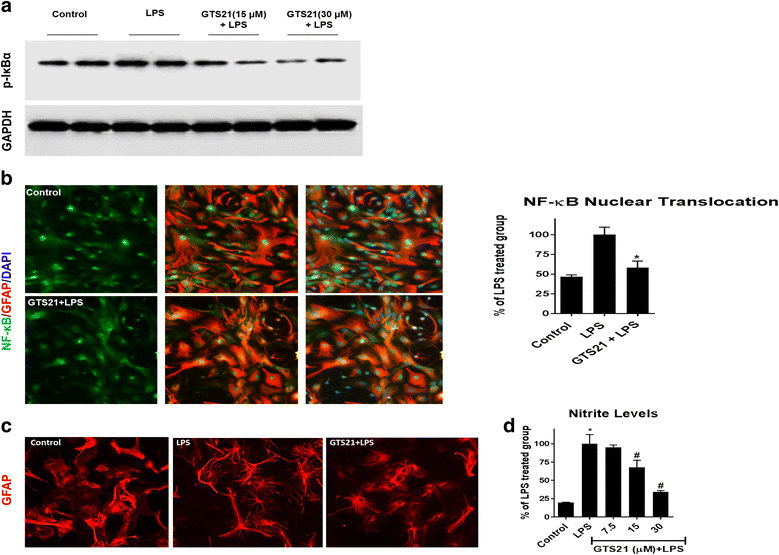
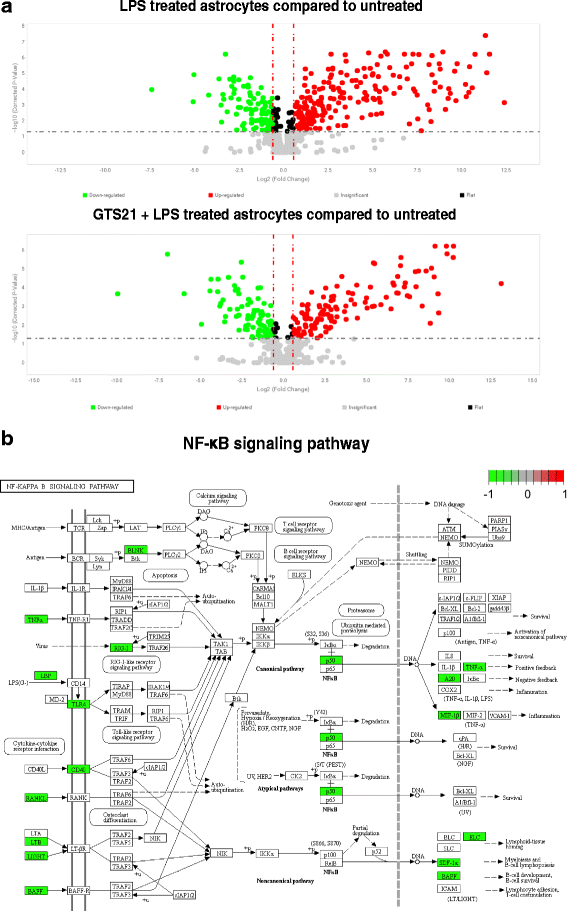
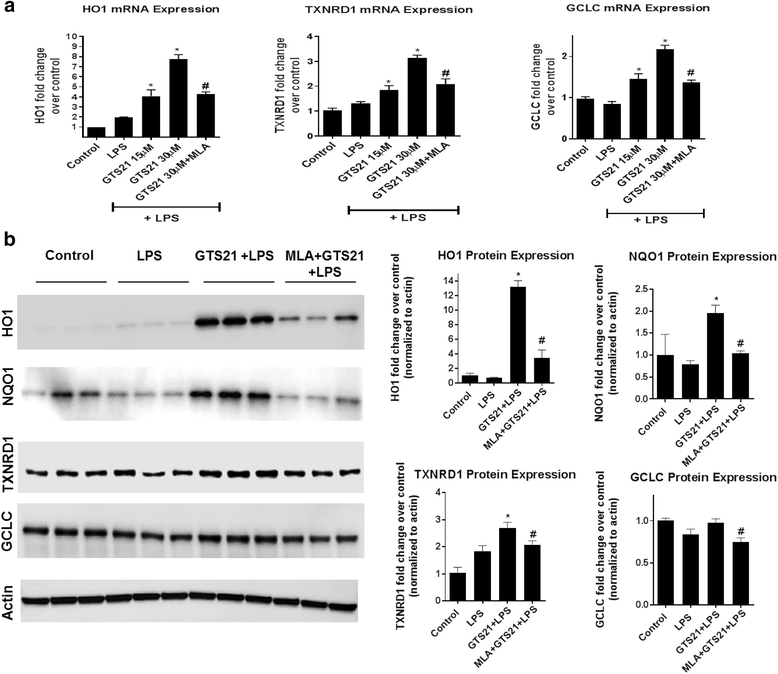
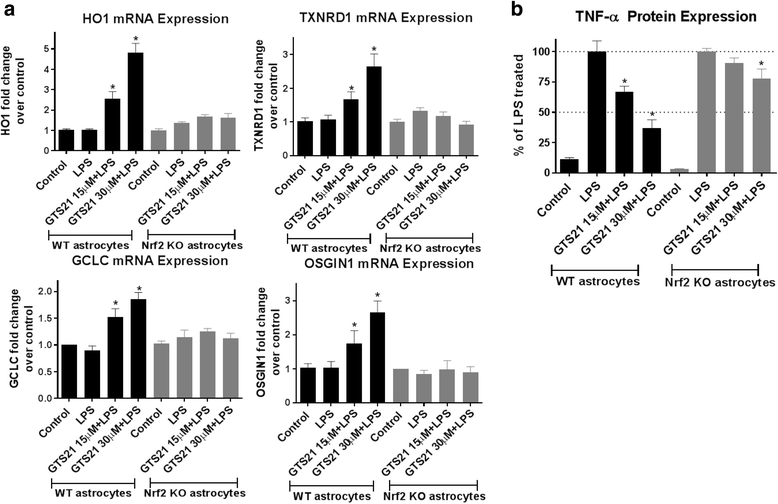
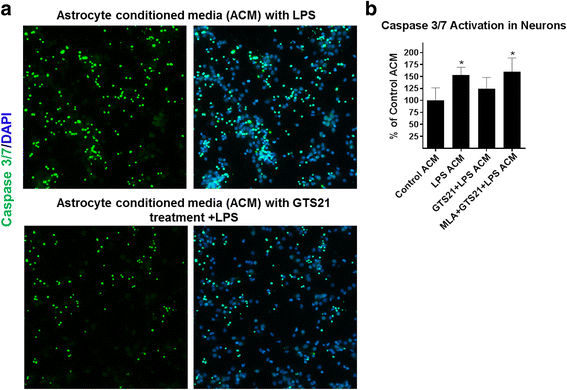
Results: Astrocytes treated with the α7 nAChR partial agonist (GTS21) showed significantly reduced LPS-mediated secretion of inflammatory cytokines and this effect was reversed by the α7 nAChR antagonist methyllycaconitine (MLA) and by knockdown of α7 nAChR expression with a short hairpin RNA. Further, α7 nAChR activation blocked LPS-mediated NF-κB nuclear translocation indicating that the observed anti-inflammatory effect may be mediated through inhibition of the NF-κB pathway. Treatment with GTS21 also upregulated canonical Nrf2 antioxidant genes and proteins suggesting antioxidant properties of α7 nAChR in astrocytes. Using an astrocyte conditioned media approach, we demonstrated reduction in neuronal apoptosis when astrocytes were pretreated with GTS21. Finally, in an in vivo neuroinflammation model using LPS in NF-κB luciferase reporter mice, we demonstrated reduction in LPS-induced NF-κB activity and pro-inflammatory cytokines with GTS21 treatment in brain tissue.
Conclusion: Our results suggest that activating astroglial α7 nAChRs may have a role in neuroprotection by decreasing inflammation and oxidative stress, and therefore could have therapeutic implication for disease modifying treatments of neurodegenerative diseases.
Keywords: Astrocytes; NF-κB pathway; Nrf2 pathway; α7 nicotinic acetylcholine receptors.
Conflict of interest statement
Ethics approval and consent to participate
All procedures involving animals were approved by the Biogen Institutional Animal Care and Use Committee (IACUC), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Consent for publication
Not applicable.
Competing interests
This research was conducted as a partial fulfillment of Dr. Patel’s doctoral program requirement at Northeastern University in Boston, MA, in collaboration with Biogen. Dr. Dunah, Ms. Ryan, and Ms. McIntyre are employees of Biogen. Dr. Patel is currently a full-time employee of Abbvie. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Del Barrio L, Martin-de-Saavedra MD, Romero A, Parada E, Egea J, Avila J, McIntosh JM, Wonnacott S, Lopez MG. Neurotoxicity induced by okadaic acid in the human neuroblastoma SH-SY5Y line can be differentially prevented by α7 and β2* nicotinic stimulation. Toxicol Sci. 2011;123:193–205. doi: 10.1093/toxsci/kfr163. - DOI - PubMed
-
- Parada E, Egea J, Romero A, del Barrio L, Garcia AG, Lopez MG. Poststress treatment with PNU282987 can rescue SH-SY5Y cells undergoing apoptosis via alpha7 nicotinic receptors linked to a Jak2/Akt/HO-1 signaling pathway. Free Radic Biol Med. 2010;49:1815–1821. doi: 10.1016/j.freeradbiomed.2010.09.017. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

