Opioid-induced rewards, locomotion, and dopamine activation: A proposed model for control by mesopontine and rostromedial tegmental neurons
- PMID: 28951251
- PMCID: PMC5730464
- DOI: 10.1016/j.neubiorev.2017.09.022
Opioid-induced rewards, locomotion, and dopamine activation: A proposed model for control by mesopontine and rostromedial tegmental neurons
Abstract
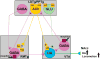
Opioids, such as morphine or heroin, increase forebrain dopamine (DA) release and locomotion, and support the acquisition of conditioned place preference (CPP) or self-administration. The most sensitive sites for these opioid effects in rodents are in the ventral tegmental area (VTA) and rostromedial tegmental nucleus (RMTg). Opioid inhibition of GABA neurons in these sites is hypothesized to lead to arousing and rewarding effects through disinhibition of VTA DA neurons. We review findings that the laterodorsal tegmental (LDTg) and pedunculopontine tegmental (PPTg) nuclei, which each contain cholinergic, GABAergic, and glutamatergic cells, are important for these effects. LDTg and/or PPTg cholinergic inputs to VTA mediate opioid-induced locomotion and DA activation via VTA M5 muscarinic receptors. LDTg and/or PPTg cholinergic inputs to RMTg also modulate opioid-induced locomotion. Lesions or inhibition of LDTg or PPTg neurons reduce morphine-induced increases in forebrain DA release, acquisition of morphine CPP or self-administration. We propose a circuit model that links VTA and RMTg GABA with LDTg and PPTg neurons critical for DA-dependent opioid effects in drug-naïve rodents.
Keywords: Acetylcholine; Addiction; GABA; Glutamate; Laterodorsal tegmental nucleus; Morphine; Pedunculopontine tegmental nucleus; Reward.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


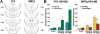
References
-
- Amalric M, Koob GF. Low doses of methylnaloxonium in the nucleus accumbens antagonize hyperactivity induced by heroin in the rat. Pharmacol. Biochem. Behav. 1985;23:411–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

