When is a randomised controlled trial health equity relevant? Development and validation of a conceptual framework
- PMID: 28951402
- PMCID: PMC5623521
- DOI: 10.1136/bmjopen-2016-015815
When is a randomised controlled trial health equity relevant? Development and validation of a conceptual framework
Abstract
Background: Randomised controlled trials can provide evidence relevant to assessing the equity impact of an intervention, but such information is often poorly reported. We describe a conceptual framework to identify health equity-relevant randomised trials with the aim of improving the design and reporting of such trials.
Methods: An interdisciplinary and international research team engaged in an iterative consensus building process to develop and refine the conceptual framework via face-to-face meetings, teleconferences and email correspondence, including findings from a validation exercise whereby two independent reviewers used the emerging framework to classify a sample of randomised trials.
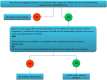
Results: A randomised trial can usefully be classified as 'health equity relevant' if it assesses the effects of an intervention on the health or its determinants of either individuals or a population who experience ill health due to disadvantage defined across one or more social determinants of health. Health equity-relevant randomised trials can either exclusively focus on a single population or collect data potentially useful for assessing differential effects of the intervention across multiple populations experiencing different levels or types of social disadvantage. Trials that are not classified as 'health equity relevant' may nevertheless provide information that is indirectly relevant to assessing equity impact, including information about individual level variation unrelated to social disadvantage and potentially useful in secondary modelling studies.
Conclusion: The conceptual framework may be used to design and report randomised trials. The framework could also be used for other study designs to contribute to the evidence base for improved health equity.
Keywords: equity; framework; health; randomized controlled trials.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- World Health Organization. Rio political declaration on social determinants of health . Geneva (CH): World Health Organization, 2011. http://www.who.int/sdhconference/declaration/en/ (accessed 10 Dec 2016).
-
- Statistics Canada. Life Expectancy. 2015. http://www.statcan.gc.ca/pub/89-645-x/2010001/life-expectancy-esperance-... (accessed 10 Dec 2016).
-
- Eyal N. Inequalities in health: concepts, measures, and ethics: Oxford University Press, 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources