Inflammatory Monocytes Promote Perineural Invasion via CCL2-Mediated Recruitment and Cathepsin B Expression
- PMID: 28951461
- PMCID: PMC5831809
- DOI: 10.1158/0008-5472.CAN-17-1612
Inflammatory Monocytes Promote Perineural Invasion via CCL2-Mediated Recruitment and Cathepsin B Expression
Abstract
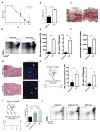
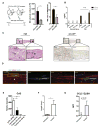
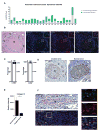
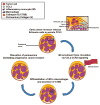
Perineural invasion (PNI) is an ominous event strongly linked to poor clinical outcome. Cells residing within peripheral nerves collaborate with cancer cells to enable PNI, but the contributing conditions within the tumor microenvironment are not well understood. Here, we show that CCR2-expressing inflammatory monocytes (IM) are preferentially recruited to sites of PNI, where they differentiate into macrophages and potentiate nerve invasion through a cathepsin B-mediated process. A series of adoptive transfer experiments with genetically engineered donors and recipients demonstrated that IM recruitment to nerves was driven by CCL2 released from Schwann cells at the site of PNI, but not CCL7, an alternate ligand for CCR2. Interruption of either CCL2-CCR2 signaling or cathepsin B function significantly impaired PNI in vivo Correlative studies in human specimens demonstrated that cathepsin B-producing macrophages were enriched in invaded nerves, which was associated with increased local tumor recurrence. These findings deepen our understanding of PNI pathogenesis and illuminate how PNI is driven in part by corruption of a nerve repair program. Further, they support the exploration of inhibiting IM recruitment and function as a targeted therapy for PNI. Cancer Res; 77(22); 6400-14. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nature reviews Cancer. 2011;11:695–707. - PubMed
-
- Amit M, Na’ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nature reviews Cancer. 2016;16:399–408. - PubMed
-
- Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3404–15. - PMC - PubMed
-
- Zeng L, Guo Y, Liang J, Chen S, Peng P, Zhang Q, et al. Perineural Invasion and TAMs in Pancreatic Ductal Adenocarcinomas: Review of the Original Pathology Reports Using Immunohistochemical Enhancement and Relationships with Clinicopathological Features. Journal of Cancer. 2014;5:754–60. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

