Wholly Rickettsia! Reconstructed Metabolic Profile of the Quintessential Bacterial Parasite of Eukaryotic Cells
- PMID: 28951473
- PMCID: PMC5615194
- DOI: 10.1128/mBio.00859-17
Wholly Rickettsia! Reconstructed Metabolic Profile of the Quintessential Bacterial Parasite of Eukaryotic Cells
Abstract
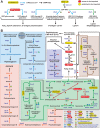
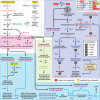
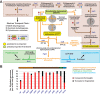
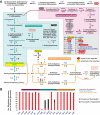
Reductive genome evolution has purged many metabolic pathways from obligate intracellular Rickettsia (Alphaproteobacteria; Rickettsiaceae). While some aspects of host-dependent rickettsial metabolism have been characterized, the array of host-acquired metabolites and their cognate transporters remains unknown. This dearth of information has thwarted efforts to obtain an axenic Rickettsia culture, a major impediment to conventional genetic approaches. Using phylogenomics and computational pathway analysis, we reconstructed the Rickettsia metabolic and transport network, identifying 51 host-acquired metabolites (only 21 previously characterized) needed to compensate for degraded biosynthesis pathways. In the absence of glycolysis and the pentose phosphate pathway, cell envelope glycoconjugates are synthesized from three imported host sugars, with a range of additional host-acquired metabolites fueling the tricarboxylic acid cycle. Fatty acid and glycerophospholipid pathways also initiate from host precursors, and import of both isoprenes and terpenoids is required for the synthesis of ubiquinone and the lipid carrier of lipid I and O-antigen. Unlike metabolite-provisioning bacterial symbionts of arthropods, rickettsiae cannot synthesize B vitamins or most other cofactors, accentuating their parasitic nature. Six biosynthesis pathways contain holes (missing enzymes); similar patterns in taxonomically diverse bacteria suggest alternative enzymes that await discovery. A paucity of characterized and predicted transporters emphasizes the knowledge gap concerning how rickettsiae import host metabolites, some of which are large and not known to be transported by bacteria. Collectively, our reconstructed metabolic network offers clues to how rickettsiae hijack host metabolic pathways. This blueprint for growth determinants is an important step toward the design of axenic media to rescue rickettsiae from the eukaryotic cell.IMPORTANCE A hallmark of obligate intracellular bacteria is the tradeoff of metabolic genes for the ability to acquire host metabolites. For species of Rickettsia, arthropod-borne parasites with the potential to cause serious human disease, the range of pilfered host metabolites is unknown. This information is critical for dissociating rickettsiae from eukaryotic cells to facilitate rickettsial genetic manipulation. In this study, we reconstructed the Rickettsia metabolic network and identified 51 host metabolites required to compensate patchwork Rickettsia biosynthesis pathways. Remarkably, some metabolites are not known to be transported by any bacteria, and overall, few cognate transporters were identified. Several pathways contain missing enzymes, yet similar pathways in unrelated bacteria indicate convergence and possible novel enzymes awaiting characterization. Our work illuminates the parasitic nature by which rickettsiae hijack host metabolism to counterbalance numerous disintegrated biosynthesis pathways that have arisen through evolution within the eukaryotic cell. This metabolic blueprint reveals what a Rickettsia axenic medium might entail.
Keywords: Rickettsia; evolution; host-parasite relationship; host-pathogen interactions; intracellular parasites; metabolic modeling; phylogenetic analysis; phylogenomics.
Copyright © 2017 Driscoll et al.
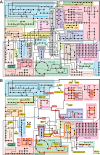
Figures


References
-
- Gillespie JJ, Nordberg EK, Azad AA, Sobral BW. 2012. Phylogeny and comparative genomics: the shifting landscape in the genomics era, p 84–141. In Azad AF, Palmer GH (ed), Intracellular pathogens II: rickettsiales. ASM Press, Washington, DC.
-
- Szokoli F, Castelli M, Sabaneyeva E, Schrallhammer M, Krenek S, Doak TG, Berendonk TU, Petroni G. 2016. Disentangling the taxonomy of Rickettsiales and description of two novel symbionts (“Candidatus Bealeia paramacronuclearis” and “Candidatus Fokinia cryptica”) sharing the cytoplasm of the ciliate protist Paramecium biaurelia. Appl Environ Microbiol 82:7236–7247. doi:10.1128/AEM.02284-16. - DOI - PMC - PubMed
-
- Beier-Sexton M, Driscoll TP, Azad AF, Gillespie JJ. 2015. The family Rickettsiaceae, p 547–566. In Goldman E, Green LH (ed), Practical handbook of microbiology, 3rd edition. CRC Press, Boca Raton, FL.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

