A surface-stabilized ozonide triggers bromide oxidation at the aqueous solution-vapour interface
- PMID: 28951540
- PMCID: PMC5615067
- DOI: 10.1038/s41467-017-00823-x
A surface-stabilized ozonide triggers bromide oxidation at the aqueous solution-vapour interface
Abstract
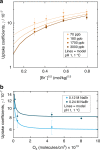
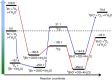
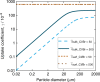
Oxidation of bromide in aqueous environments initiates the formation of molecular halogen compounds, which is important for the global tropospheric ozone budget. In the aqueous bulk, oxidation of bromide by ozone involves a [Br•OOO-] complex as intermediate. Here we report liquid jet X-ray photoelectron spectroscopy measurements that provide direct experimental evidence for the ozonide and establish its propensity for the solution-vapour interface. Theoretical calculations support these findings, showing that water stabilizes the ozonide and lowers the energy of the transition state at neutral pH. Kinetic experiments confirm the dominance of the heterogeneous oxidation route established by this precursor at low, atmospherically relevant ozone concentrations. Taken together, our results provide a strong case of different reaction kinetics and mechanisms of reactions occurring at the aqueous phase-vapour interface compared with the bulk aqueous phase.Heterogeneous oxidation of bromide in atmospheric aqueous environments has long been suspected to be accelerated at the interface between aqueous solution and air. Here, the authors provide spectroscopic, kinetic and theoretical evidence for a rate limiting, surface active ozonide formed at the interface.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Oum KW, Lakin MJ, Finlayson-Pitts BJ. Bromine activation in the troposphere by the dark reaction of O-3 with seawater ice. Geophys. Res. Lett. 1998;25:3923–3926. doi: 10.1029/1998GL900078. - DOI
-
- De Haan DO, et al. Heterogeneous chemistry in the troposphere: experimental approaches and applications to the chemistry of sea salt particles. Int. Rev. Phys. Chem. 1999;18:343–385. doi: 10.1080/014423599229910. - DOI
-
- Parrella JP, et al. Tropospheric bromine chemistry: implications for present and pre-industrial ozone and mercury. Atmos. Chem. Phys. 2012;12:6723–6740. doi: 10.5194/acp-12-6723-2012. - DOI
-
- Schmidt JA, et al. Modeling the observed tropospheric BrO background: Importance of multiphase chemistry and implications for ozone, OH, and mercury. J. Geophys. Res. 2016;121:11819–11835.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

