High-Affinity Functional Fluorescent Ligands for Human β-Adrenoceptors
- PMID: 28951558
- PMCID: PMC5614969
- DOI: 10.1038/s41598-017-12468-3
High-Affinity Functional Fluorescent Ligands for Human β-Adrenoceptors
Abstract
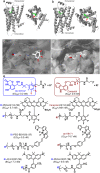
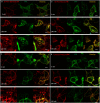
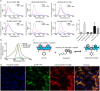
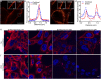
Visualization of the G-protein coupled receptor (GPCR) is of great importance for studying its function in a native cell. We have synthesized a series of red-emitting fluorescent probes targeting β-adrenergic receptor (βAR) that are compatible with confocal and Stimulated Emission Depletion (STED) microscopy as well as with Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) binding assay in living cells. The probe based on the agonist BI-167107 and fluorescent dye KK114 demonstrates nanomolar binding affinity and up to nine-fold β2AR selectivity over β1AR. Carazolol-derived probes are fluorogenic and allow no-wash imaging experiments. STED microscopy of β2ARs stained at the native expression level on pancreatic CAPAN cells provides two-fold improvement in lateral optical resolution over confocal mode and reveals the formation of receptor microdomains. These probes retain their functional (agonist or antagonist) properties, allowing simultaneous modulation of cyclic adenosine monophosphate (cAMP) levels and receptor internalization as well as imaging receptor localization.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Ross EM, Maguire ME, Sturgill TW, Biltonen RL, Gilman AG. Relationship between Beta-Adrenergic-Receptor and Adenylate-Cyclase - Studies of Ligand-Binding and Enzyme-Activity in Purified Membranes of S49 Lymphoma-Cells. J. Biol. Chem. 1977;252:5761–5775. - PubMed
-
- Delean A, Stadel JM, Lefkowitz RJ. A Ternary Complex Model Explains the Agonist-Specific Binding-Properties of the Adenylate Cyclase-Coupled Beta-Adrenergic-Receptor. J. Biol. Chem. 1980;255:7108–7117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

