Neuronal-Glial Interactions Maintain Chronic Neuropathic Pain after Spinal Cord Injury
- PMID: 28951789
- PMCID: PMC5603132
- DOI: 10.1155/2017/2480689
Neuronal-Glial Interactions Maintain Chronic Neuropathic Pain after Spinal Cord Injury
Abstract
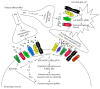
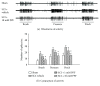
The hyperactive state of sensory neurons in the spinal cord enhances pain transmission. Spinal glial cells have also been implicated in enhanced excitability of spinal dorsal horn neurons, resulting in pain amplification and distortions. Traumatic injuries of the neural system such as spinal cord injury (SCI) induce neuronal hyperactivity and glial activation, causing maladaptive synaptic plasticity in the spinal cord. Recent studies demonstrate that SCI causes persistent glial activation with concomitant neuronal hyperactivity, thus providing the substrate for central neuropathic pain. Hyperactive sensory neurons and activated glial cells increase intracellular and extracellular glutamate, neuropeptides, adenosine triphosphates, proinflammatory cytokines, and reactive oxygen species concentrations, all of which enhance pain transmission. In addition, hyperactive sensory neurons and glial cells overexpress receptors and ion channels that maintain this enhanced pain transmission. Therefore, post-SCI neuronal-glial interactions create maladaptive synaptic circuits and activate intracellular signaling events that permanently contribute to enhanced neuropathic pain. In this review, we describe how hyperactivity of sensory neurons contributes to the maintenance of chronic neuropathic pain via neuronal-glial interactions following SCI.
Figures

References
-
- Merskey H., Bogduk N. Classification of Chronic Pain. Seattle: IASP Press; 1994.
-
- Baron R. Peripheral neuropathic pain. The Clinical Journal of Pain. 2000;16(2):S12–S20. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

