Filtering out parasites: sand crabs (Lepidopa benedicti) are infected by more parasites than sympatric mole crabs (Emerita benedicti)
- PMID: 28951818
- PMCID: PMC5611900
- DOI: 10.7717/peerj.3852
Filtering out parasites: sand crabs (Lepidopa benedicti) are infected by more parasites than sympatric mole crabs (Emerita benedicti)
Abstract
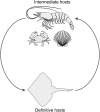
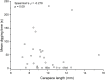
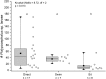
Two digging decapod crustaceans, the sand crab species Lepidopa benedicti and the mole crab species Emerita benedicti, both live in the swash zone of fine sand beaches. They were examined for two parasites that infect decapod crustaceans in the region, an unidentified nematode previously shown to infect L. benedicti, and cestode tapeworm larvae, Polypocephalus sp., previously shown to infect shrimp (Litopenaeus setiferus). Lepidopa benedicti were almost always infected with both parasite species, while E. benedicti were rarely infected with either parasite species. This difference in infection pattern suggests that tapeworms are ingested during sediment feeding in L. benedicti, which E. benedicti avoid by filter feeding. Larger L. benedicti had more Polypocephalus sp. larvae. The thoracic ganglia, which make up the largest proportion of neural tissue, contained the largest numbers of Polypocephalus sp. larvae. Intensity of Polypocephalus sp. infection was not correlated with how long L. benedicti remained above sand in behavioural tests, suggesting that Polypocephalus sp. do not manipulate the sand crabs in a way that facilitates trophic transmission of the parasite. Litopenaeus setiferus may be a primary host for Polypocephalus sp., and L. benedict may be a secondary, auxiliary host.
Keywords: Cestode; Crustacean; Digging; Hippoidea; Nematode; Parasite; Parasite manipulation of behavior.
Conflict of interest statement
The author declares there are no competing interests.
Figures







Similar articles
-
Polypocephalus sp. infects the nervous system and increases activity of commercially harvested white shrimp (Litopenaeus setiferus).J Parasitol. 2011 Oct;97(5):755-9. doi: 10.1645/GE-2749.1. Epub 2011 Mar 28. J Parasitol. 2011. PMID: 21506800
-
Nematodes infect, but do not manipulate digging by, sand crabs, Lepidopa benedicti.Integr Comp Biol. 2014 Jul;54(2):101-7. doi: 10.1093/icb/icu064. Epub 2014 Jun 10. Integr Comp Biol. 2014. PMID: 24916475 Free PMC article.
-
Burrowing abilities and swash behavior of three crabs, Emerita analoga Stimpson, Blepharipoda occidentalis Randall, and Lepidopa californica Efford (Anomura, Hippoidea), of exposed sandy beaches.J Exp Mar Biol Ecol. 2000 Dec 20;255(2):229-245. doi: 10.1016/s0022-0981(00)00294-x. J Exp Mar Biol Ecol. 2000. PMID: 11108854
-
Breeding biology of the intertidal sand crab, Emerita (Decapoda: Anomura).Adv Mar Biol. 2003;46:91-182. doi: 10.1016/s0065-2881(03)46003-3. Adv Mar Biol. 2003. PMID: 14601412 Review.
-
Human behaviour and the epidemiology of parasitic zoonoses.Int J Parasitol. 2005 Oct;35(11-12):1319-31. doi: 10.1016/j.ijpara.2005.06.004. Epub 2005 Jul 20. Int J Parasitol. 2005. PMID: 16102769 Review.
References
-
- Adamson ML, Caira JN. Evolutionary factors influencing the nature of parasite specificity. Parasitology. 2011;109:S85–S95. - PubMed
-
- Bullock TH. How are more complex brains different? One view and an agenda for comparative neurobiology. Brain, Behavior and Evolution. 1993;41:88–96. - PubMed
-
- Bullock TH. Neuroethology has pregnant agendas. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2004;185:291–295. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

