Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis
- PMID: 28951820
- PMCID: PMC5605733
- DOI: 10.1016/j.molmet.2017.06.019
Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis
Abstract
Background: Plasma insulin levels are predominantly the product of the morphological mass of insulin producing beta cells in the pancreatic islets of Langerhans and the functional status of each of these beta cells. Thus, deficiency in either beta cell mass or function, or both, can lead to insufficient levels of insulin, resulting in hyperglycemia and diabetes. Nonetheless, the precise contribution of beta cell mass and function to the pathogenesis of diabetes as well as the underlying mechanisms are still unclear. In the past, this was largely due to the restricted number of technologies suitable for studying the scarcely accessible human beta cells. However, in recent years, a number of new platforms have been established to expand the available techniques and to facilitate deeper insight into the role of human beta cell mass and function as cause for diabetes and as potential treatment targets.
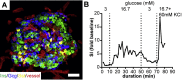
Scope of review: This review discusses the current knowledge about contribution of human beta cell mass and function to different stages of type 1 and type 2 diabetes pathogenesis. Furthermore, it highlights standard and newly developed technological platforms for the study of human beta cell biology, which can be used to increase our understanding of beta cell mass and function in human glucose homeostasis.
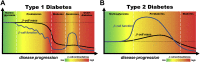
Major conclusions: In contrast to early disease models, recent studies suggest that in type 1 and type 2 diabetes impairment of beta cell function is an early feature of disease pathogenesis while a substantial decrease in beta cell mass occurs more closely to clinical manifestation. This suggests that, in addition to beta cell mass replacement for late stage therapies, the development of novel strategies for protection and recovery of beta cell function could be most promising for successful diabetes treatment and prevention. The use of today's developing and wide range of technologies and platforms for the study of human beta cells will allow for a more detailed investigation of the underlying mechanisms and will facilitate development of treatment approaches to specifically target human beta cell mass and function.
Keywords: Beta cell function; Beta cell mass; Diabetes; Human; In situ; In vitro; In vivo; Islet of Langerhans; Pathogenesis.
Figures
References
-
- Eisenbarth G.S. Type I diabetes mellitus. A chronic autoimmune disease. The New England Journal of Medicine. 1986;314(21):1360–1368. - PubMed
-
- Chatenoud L., Bluestone J.A. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nature Reviews Immunology. 2007;7(8):622–632. - PubMed
-
- von Herrath M., Sanda S., Herold K. Type 1 diabetes as a relapsing-remitting disease? Nature Reviews Immunology. 2007;7(12):988–994. - PubMed
-
- van Belle T.L., Coppieters K.T., von Herrath M.G. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiological Reviews. 2011;91(1):79–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

