Endoplasmic reticulum stress and eIF2α phosphorylation: The Achilles heel of pancreatic β cells
- PMID: 28951826
- PMCID: PMC5605732
- DOI: 10.1016/j.molmet.2017.06.001
Endoplasmic reticulum stress and eIF2α phosphorylation: The Achilles heel of pancreatic β cells
Abstract
Background: Pancreatic β cell dysfunction and death are central in the pathogenesis of most if not all forms of diabetes. Understanding the molecular mechanisms underlying β cell failure is important to develop β cell protective approaches.
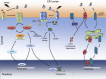
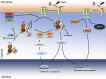
Scope of review: Here we review the role of endoplasmic reticulum stress and dysregulated endoplasmic reticulum stress signaling in β cell failure in monogenic and polygenic forms of diabetes. There is substantial evidence for the presence of endoplasmic reticulum stress in β cells in type 1 and type 2 diabetes. Direct evidence for the importance of this stress response is provided by an increasing number of monogenic forms of diabetes. In particular, mutations in the PERK branch of the unfolded protein response provide insight into its importance for human β cell function and survival. The knowledge gained from different rodent models is reviewed. More disease- and patient-relevant models, using human induced pluripotent stem cells differentiated into β cells, will further advance our understanding of pathogenic mechanisms. Finally, we review the therapeutic modulation of endoplasmic reticulum stress and signaling in β cells.
Major conclusions: Pancreatic β cells are sensitive to excessive endoplasmic reticulum stress and dysregulated eIF2α phosphorylation, as indicated by transcriptome data, monogenic forms of diabetes and pharmacological studies. This should be taken into consideration when devising new therapeutic approaches for diabetes.
Keywords: ATF, activating transcription factor; CHOP, C/EBP homologous protein; CRISPR, clustered regularly interspaced short palindromic repeats; CReP, constitutive repressor of eIF2α phosphorylation; Diabetes; ER, endoplasmic reticulum; ERAD, ER-associated degradation; Endoplasmic reticulum stress; GCN2, general control non-derepressible-2; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; GWAS, genome-wide association study; HNF1A, hepatocyte nuclear factor 1-α; HRI, heme-regulated inhibitor kinase; IAPP, islet amyloid polypeptide; IER3IP1, immediate early response-3 interacting protein-1; IRE1, inositol-requiring protein-1; ISR, integrated stress response; Insulin; Islet; MEHMO, mental retardation, epilepsy, hypogonadism and -genitalism, microcephaly and obesity; MODY, maturity-onset diabetes of the young; NRF2, nuclear factor, erythroid 2 like 2; PBA, 4-phenyl butyric acid; PERK, PKR-like ER kinase; PKR, protein kinase RNA; PP1, protein phosphatase 1; PPA, phenylpropenoic acid glucoside; Pancreatic β cell; Pdx1, pancreatic duodenal homeobox 1; RIDD, regulated IRE1-dependent decay; RyR2, type 2 ryanodine receptor/Ca2+ release channel; SERCA, sarcoendoplasmic reticulum Ca2+ ATPase; TUDCA, taurine-conjugated ursodeoxycholic acid derivative; UPR, unfolded protein response; WFS, Wolfram syndrome; XBP1, X-box binding protein 1; eIF2, eukaryotic translation initiation factor 2; eIF2α; hESC, human embryonic stem cell; hPSC, human pluripotent stem cell; hiPSC, human induced pluripotent stem cell; uORF, upstream open reading frame.
Figures


References
-
- IDF diabetes atlas. 2015. http://www.diabetesatlas.org/ Available from: (accessed 25.01.17) - PubMed
-
- Vaxillaire M., Bonnefond A., Froguel P. The lessons of early-onset monogenic diabetes for the understanding of diabetes pathogenesis. Best Practice & Research Clinical Endocrinology & Metabolism. 2012;26:171–187. - PubMed
-
- Flannick J., Johansson S., Njolstad P.R. Common and rare forms of diabetes mellitus: towards a continuum of diabetes subtypes. Nature Reviews Endocrinology. 2016;12:394–406. - PubMed
-
- Murphy R., Ellard S., Hattersley A.T. Clinical implications of a molecular genetic classification of monogenic β-cell diabetes. Nature Clinical Practice Endocrinology & Metabolism. 2008;4:200–213. - PubMed
-
- Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8:519–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

