Cross-Talk between Glia, Neurons and Mast Cells in Neuroinflammation Associated with Parkinson's Disease
- PMID: 28952015
- PMCID: PMC5802395
- DOI: 10.1007/s11481-017-9766-1
Cross-Talk between Glia, Neurons and Mast Cells in Neuroinflammation Associated with Parkinson's Disease
Abstract
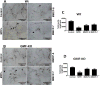
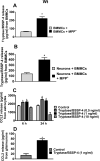
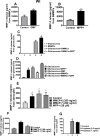
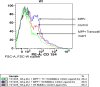
Parkinson's disease (PD) is a progressive movement disorder characterized by neuroinflammation and dopaminergic neurodegeneration in the brain. 1-methyl-4-phenylpyridinium (MPP+), a metabolite of the parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induces the release of inflammatory mediators from glial cells and neurons. Glia maturation factor (GMF), a brain proinflammatory protein, MPP+, and mast cell-derived inflammatory mediators induce neurodegeneration which eventually leads to PD. However, the precise mechanisms underlying interaction between glial cells, neurons and mast cells in PD still remain elusive. In the present study, mouse bone marrow-derived mast cells (BMMCs) and mouse fetal brain-derived mixed glia/neurons, astrocytes and neurons were incubated with MPP+, GMF and mast cell-derived inflammatory mediators mouse mast cell protease-6 (MMCP-6), MMCP-7 or tryptase/brain-specific serine protease-4 (tryptase/BSSP-4). Inflammatory mediators released from these cells in the culture medium were quantitated by enzyme-linked immunosorbent assay. Neurodegeneration was quantified by measuring total neurite outgrowth following microtubule-associated protein-2 immunocytochemistry. MPP+-induced significant neurodegeneration with reduced total neurite outgrowth. MPP+induced the release of tryptase/BSSP-4 from the mouse mast cells, and tryptase/BSSP-4 induced chemokine (C-C motif) ligand 2 (CCL2) release from astrocytes and glia/neurons. Overall our results suggest that MPP+, GMF, MMCP-6 or MMCP-7 stimulate glia/neurons, astrocytes or neurons to release CCL2 and matrix metalloproteinase-3. Additionally, CD40L expression is increased in BMMCs after incubation with MPP+ in a co-culture system consisting of BMMCs and glia/neurons. We propose that mast cell interaction with glial cells and neurons during neuroinflammation can be explored as a new therapeutic target for PD.
Keywords: 1-methyl-4-phenylpyridinium; CCL2; Cytokines; Glia maturation factor; Mast cells; Microtubule-associated protein-2; Neuroinflammation; Parkinson’s disease.
Conflict of interest statement
Authors declare that they have no competing interests.
Figures




References
-
- Bennett JL, Blanchet MR, Zhao L, Zbytnuik L, Antignano F, Gold M, Kubes P, McNagny KM. Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. J Immunol. 2009;182:5507–5514. - PubMed
-
- Cottrell GS, Amadesi S, Schmidlin F, Bunnett N. Protease-activated receptor 2: activation, signalling and function. Biochem Soc Trans. 2003;31:1191–1197. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

